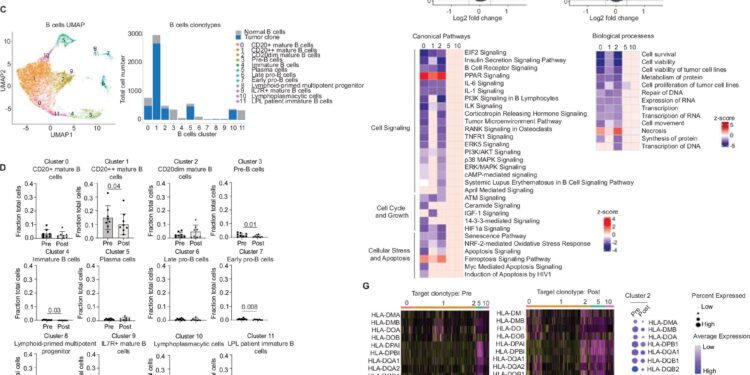
Single-cell RNA-seq analysis reveals vaccine-associated reduction in LPL B cells, but not plasma cell-like subpopulations in bone marrow. Credit: Nature Communications (2024). DOI: 10.1038/s41467-024-50880-2
A team of researchers from City of Hope and the University of Texas MD Anderson Cancer Center reported safety and efficacy results from a phase 1 trial that tested a personalized vaccine to treat lymphoplasmacytic lymphoma, a rare, slow-growing type of blood cancer, according to a study recently published in Nature Communications.
The current approach to treating lymphoplasmacytic lymphoma is based on active monitoring of the patient’s possible symptoms. The median time from diagnosis to progression of symptoms (fever, night sweats, weight loss, and fatigue) requiring chemotherapy is 3.5 years.
“By intervening early with the vaccine, we nearly doubled the time to disease-free progression, to an average of just under seven years,” said Larry Kwak, MD, PhD, director of City of Hope’s Toni Stephenson Lymphoma Center within the Hematologic Malignancies Research Institute, who developed the vaccine and is the study’s corresponding author.
“In addition to being effective, the vaccine appears to be safe. It does not have any of the serious side effects associated with other types of common cancer treatments.”
Toxicity among trial participants was also limited, Dr. Kwak said. That’s because the vaccine uses patient-specific biological components, called tumor neoantigens, that can help the body mount an immune response against a particular tumor type.
The clinical trial, led by Dr. Anderson, involved nine patients who were able to tolerate the treatment without negative side effects. After a median follow-up of 7.5 years, all patients had stable disease and more than half had not progressed to a symptomatic state.
“Using a sophisticated technology called single-cell sequencing, we were able to see that these personalized vaccines activated T cells in the tumor microenvironment, which help destroy tumor cells,” said Dr. Kwak, who is also deputy director of City of Hope’s Comprehensive Cancer Center and the Dr. Michael Friedman Professor of Translational Medicine. “In addition, we found that tumor cells rely on myeloid cell signaling to survive, which was something we didn’t know before, and the vaccine also reduced that pro-tumor signaling.”
According to Dr. Kwak, disease progression was halted and one patient experienced a slight decrease in tumor size. The research teams believe this is because two different subtypes of cells drive lymphoplasmacytic lymphoma tumors (mature B cells and plasma cells) and the vaccine only had an effect on the B cell population.
“What we can take away from this observation is that when we do the next phase of the clinical trial, we will want to combine the vaccine with another agent that has a more direct effect on plasma cells, such as a monoclonal antibody,” he said.
Dr. Kwak and his research team are working on the next generation of the vaccine for a potential clinical trial that could take place at City of Hope. Under a sponsored research agreement with Renhaim Inc., Dr. Kwak and his team plan to adapt the vaccine to an mRNA platform, which has become a new frontier for vaccine production since the original was developed using DNA nearly a decade ago. The team is also exploring other therapies to combine with the neoantigen for greater vaccine efficacy. Dr. Kwak is a paid consultant to Renhaim Inc.
The neoantigen vaccine research builds on three decades of investigations by Dr. Kwak. In 2011, Dr. Kwak and colleagues published research in the journal Journal of Clinical Oncology on the first iteration of the neoantigen vaccine. It was a protein-based vaccine against follicular lymphoma that showed positive results.
“This was actually one of the first positive cancer vaccine trials in this space, but 15 years ago, no pharmaceutical company was interested in talking to us about a personalized vaccine,” Dr. Kwak said. He noted that the “one drug for one patient” model was a hard sell until CAR T-cell therapies set a precedent for individualized cancer drugs.
“Today, they are much more open to this idea. I think the future of cancer vaccines really lies in this type of setting, where we have demonstrated the efficacy of early intervention as a way to prolong and perhaps even prevent progression to symptomatic disease.”
More information:
Szymon J. Szymura et al, Personalized neoantigen vaccines as early intervention in untreated patients with lymphoplasmacytic lymphoma: a nonrandomized phase 1 trial, Nature Communications (2024). DOI: 10.1038/s41467-024-50880-2
Provided by City of Hope National Medical Center
Quote:Vaccine experts report positive results from phase 1 trial of personalized lymphoplasmacytic lymphoma vaccine (2024, September 19) retrieved September 19, 2024, from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.