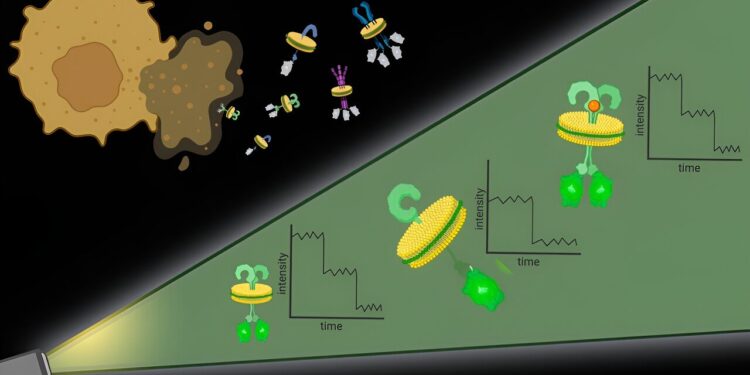
With Native-nanoBleach, nanodiscs containing target membrane proteins and their native cell membrane environment are cut from the cell membrane. Attached to each protein subunit is a molecule that fluoresces under light. Over time, exposure to light will bleach the fluorescent molecules, and the gradual decrease in fluorescence will allow researchers to count the number of protein subunits present in each nanodisc. Credit: Moitrayee Bhattacharyya
The membrane that surrounds a biological cell is not simply a barrier; it’s full of proteins involved in all kinds of critical biological functions. To truly understand what membrane proteins do and how, researchers need to know how they are organized and how they interact with each other. But discovering this information is a challenge.
Yale researchers have now developed a new microscopy method called Native-nanoBleach that overcomes key challenges in understanding the organization of membrane proteins, including the difficulty of studying these membranes without disrupting the native environment and the limitations of the resolution of the optical microscopes generally used to study them.
And to demonstrate the effectiveness of the new method, they successfully applied it to a biological puzzle – relating to which proteins are involved in the development of pancreatic cancers and how they might be targeted for treatment – which remained unresolved for decades.
They describe the new method and its benefits in a new study published in Nature Nanotechnology.
Methods typically used in the study of membrane protein organization require removing the native membrane environment surrounding the proteins and then placing the isolated proteins of interest in environments that mimic but do not fully replicate the complexity of the actual cell membrane, said Moitrayee Bhattacharyya, assistant professor of pharmacology. at the Yale School of Medicine and lead author of the study. This approach, Bhattacharyya said, removes important context since proteins interact with molecules around them.
Second, optical microscopes, commonly used to observe protein organization, do not have the resolution needed to determine whether proteins near each other actually interact or are simply neighbors on membranes.
Finally, the amount of a particular protein found in a cell membrane may be too low or too high for current study methods. In these cases, researchers must make adjustments; they must either replicate proteins that, in their natural state, are too few in number, or separate proteins from samples where there are too many. But this can again remove important context about the natural state of proteins as they sit and function in cell membranes.
“Ideally, we would have a method that would work with any endogenous level of cell membrane protein expression,” Bhattacharyya said.
To address the first challenge, the researchers used particular molecules, types of polymers, to essentially extract protein complexes with their surrounding cell membrane intact. “It’s like the cell membrane is a sheet of cookie dough and the polymers are cookie cutters,” Bhattacharyya said.
These pieces of protein surrounded by the surrounding cell membrane, called native nanodiscs, are about 10 nanometers in diameter, small enough that any proteins in the nanodisc likely interact, which addresses the second challenge. Additionally, this approach works with any cell membrane protein in any quantity, allowing researchers to observe proteins at their natural levels in native membranes.
Once the nanodiscs are generated, researchers can use a number of commonly used techniques to zero in on a particular protein of interest. They then quantify the proteins on each nanodisk using fluorescent molecules attached to them.
This is an approach that provides high spatial resolution without requiring specialized hardware, Bhattacharyya said.
“This work presents a new technique for understanding how membrane proteins, which represent approximately 60% of drug targets, assemble into functional units on or within the native lipid bilayer,” said Gerard Walker, co-first author of the study. article and graduate student. in Bhattacharyya’s laboratory.
To demonstrate how this method could be applied, the researchers embarked on a decades-long debate in biology. A protein called KRas is mutated in more than 90% of human pancreatic cancers, attracting immense clinical and therapeutic interest. The question of whether KRas subunits come together to form dimers (two units) or oligomers (more than two units) on cell membranes remains the focus of long-standing research.
However, studies have produced conflicting results. Animal and cellular studies, which lack detailed molecular resolution, show that KRas units cluster on cell membranes. Meanwhile, biophysical analyses, which do not preserve the native membrane around the proteins, have revealed remnants of KRas in single units, or monomers.
“With our method, we get the best of both worlds,” Bhattacharyya said. “We retain the native membrane environment and we have very high single spatial and molecular resolution. When we applied our method, we found that KRas exists as dimers and monomers in similar amounts. But when KRas is mutated, as in pancreatic cancers, dimers increase and monomers decrease.
The discovery highlights the importance of the native cell membrane for understanding membrane proteins and identifies a target – reducing KRas dimerization – for cancer treatment. This is just one of many ways this method could be used to understand the role of membrane protein organization in disease, Bhattacharyya said.
“It’s truly gratifying to see Native-nanoBleach already successfully applied to a wide variety of pressing biological questions in Bhattacharyya’s lab and beyond,” said Caroline Brown, co-first author of the study and PhD. ‘a Ph.D. candidate in the lab of co-author Kallol Gupta, assistant professor of cell biology.
Membrane proteins make up a third of all proteins in the human body and this approach can be used to study any of them, Bhattacharyya said.
“It’s a general technique,” she said. “There really are no limits.”
In the future, Bhattacharyya and his colleagues hope to extend this approach to study the organization of proteins in the membranes of various organelles, structures such as mitochondria, contained within cells.
More information:
Gerard Walker et al, Oligomeric organization of membrane proteins from native membranes at unique nanoscale spatial and molecular resolution, Nature Nanotechnology (2023). DOI: 10.1038/s41565-023-01547-4
Provided by Yale University
Quote: Using molecular cookie cutters to visualize the organization of membrane proteins (December 21, 2023) retrieved on December 21, 2023 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.