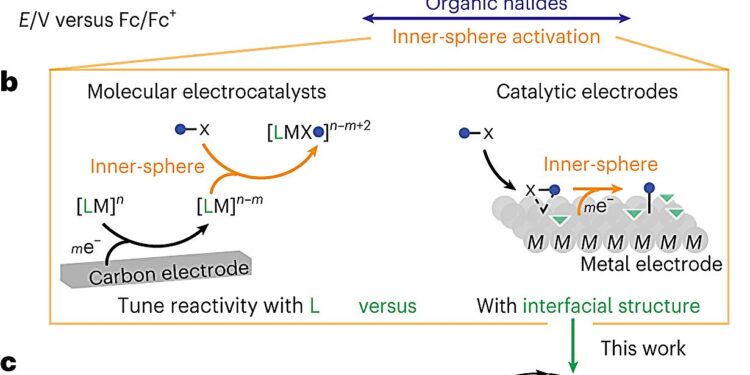
Electrocatalysis allows easy activation of organic halides. a, Selective reductive activation of organic halides over aldehydes and ketones is limited due to the similar reduction potentials accessible via OSET. b, Reductive activation of organic halides in the inner sphere via molecular electrocatalysis or catalytic electrodes drives the reduction potential of organic halides toward more positive potentials. c, This work reveals the interfacial structure of Ag catalytic electrodes that enables selective electrophilic coupling. Credit: Natural catalysis (2024). DOI: 10.1038/s41929-023-01073-5
As the world moves away from gas and toward electricity as a greener energy source, the to-do list goes beyond cars. The vast global manufacturing network that makes everything from our batteries to our fertilizers must also change course.
A study by University of Chicago chemists has discovered a way to use electricity to stimulate a type of chemical reaction often used in the synthesis of new candidates for pharmaceutical drugs.
Published on January 2 in Natural catalysisThe research represents a breakthrough in the field of electrochemistry and shows the way forward for designing and controlling reactions and making them more sustainable.
“What we want to do is understand what is happening at the fundamental level at the electrode interface and use that to predict and design more efficient chemical reactions,” said Anna Wuttig, Neubauer Family Assistant Professor at UChicago and lead author of the article. “This is a step toward that eventual goal.”
Chemical complexity
In some chemical reactions, electricity can increase production, and because you can get the necessary electricity from renewable sources, it could help make the global chemical industry greener.
But electrochemistry, as this field is called, is particularly complex. There is a lot that scientists don’t know about molecular interactions, not least because you have to insert a solid conductor (an electrode) into the mixture to provide the electricity, which means that the molecules interact with that electrode as well as ‘between them. For a scientist trying to unravel what roles each molecule plays and in what order, this makes an already complicated process even more complicated.
But Wuttig wants to turn it into an advantage. “What if you thought of this as electrochemistry providing us with a unique design lever that is not possible in any other system?” she says.
In this case, she and her team focused on the surface of the electrode that provides the electricity needed for the reaction.
“There have been hints that the surface itself is catalytic, that it plays a role,” Wuttig said, “but we don’t know how to systematically control these interactions at the molecular level.”
They tinkered with a type of reaction commonly used in making medical chemicals, to form a bond between two carbon atoms.
According to theoretical predictions, when this reaction is carried out using electricity, the reaction efficiency should be 100%, that is, all the input molecules are transformed into a single new substance. But when you actually perform the reaction in the lab, the yield is lower.
The team believed that the presence of the electrode moved certain molecules away from where they were needed during the reaction. They discovered that adding a key ingredient could help: a chemical known as Lewis acid added to the liquid solution redirected these molecules.
“You get almost a clear reaction,” Wuttig said.
Catalyze change
Additionally, the team was able to use special imaging techniques to observe reactions taking place at the molecular level. “You can see that the presence of the modulator has a profound effect on the interfacial structure,” she said. “It allows us to visualize and understand what’s going on, rather than looking at it as a black box.”
This is a crucial step, Wuttig said, because it shows the way forward to being able to not only use the electrode in chemistry, but also predict and control its effects.
Another advantage is that the electrode can be reused for more reactions. (In most reactions, the catalyst is dissolved in the liquid and is removed during the purification process to obtain the final product).
“This is a step towards a sustainable synthesis,” she said. “In the future, my group is very excited to use these types of concepts and strategies to define and address other synthetic challenges.”
More information:
Qiu-Cheng Chen et al, Interfacial tuning of electrocatalytic Ag surfaces for fragment-based electrophilic coupling, Natural catalysis (2024). DOI: 10.1038/s41929-023-01073-5
Provided by the University of Chicago
Quote: Using electricity, scientists discover a promising new method for stimulating chemical reactions (January 2, 2024) retrieved January 3, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.