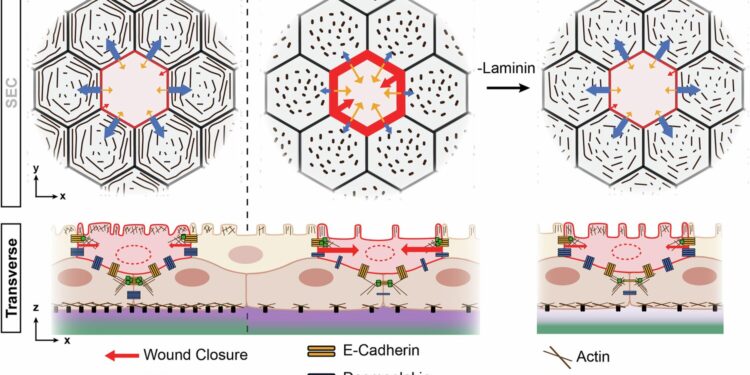
Schematic illustration of how BEC-ECM interactions influence periderm architecture and wound repair. Credit: Natural communications (2025). DOI: 10.1038/s41467-025-64040-7
Our skin protects us from everyday mechanical attacks, such as friction, cuts and impacts. A key element of this function, which provides a shield against the outside world, is the skin’s incredible ability to regenerate and heal. But where does this healing ability begin?
In a new study published in Natural communicationsAn interdisciplinary team led by the laboratories of Kaelyn Sumigray, Ph.D., and Stefania Nicoli, Ph.D., discovered that during the early stages of embryonic development, skin stem cells help form a protective skin layer that speeds healing as the embryo grows.
Their findings reveal one of the first steps in how skin stem cells learn to repair tissue – knowledge that could help design improved skin grafts for transplantation.
“We were curious about how to make skin more resistant to injury,” says Nicoli, associate professor of medicine (cardiology) and genetics at YSM and co-senior author of the study. “We discovered a mechanism that makes our skin harder, which is exciting in the sense that it is a global concept that could apply to our entire adult body.”
The researchers examined the development of zebrafish embryos, whose skin organization is similar to that of human embryos. Specifically, they analyzed the thin, transparent layer of cells that lines the folds of zebrafish fins in the embryo, structures that transform into fins during development in a process similar to limb development in mammals.
Since basal epidermal stem cells (BECs) located in the fin fold provide a stronger, more resilient region of skin as the fin begins to form, researchers have defined the mechanism of such resilience.
Resilience of embryonic zebrafish skin depends on two proteins
The researchers found that in specific regions and patterns along the fin fold, BECs expressed two proteins – collagen and laminin – that contribute to the extracellular matrix, a non-cellular network of proteins and carbohydrates that surrounds and supports cells in tissues and organs.
BECs that promote laminin reduce the formation of desmosomes (protein complexes that anchor cells together), thereby weakening cell-to-cell adhesion and allowing greater mobility during tissue damage. In contrast, collagen-promoting BECs increase desmosomes, thereby strengthening specialized junctions that ultimately hinder skin repair.
The two pathways work together to give the embryo both the space to develop a fin and then the ability to heal quickly, the researchers found.
“Stem cells have a mechanical logic to build a protective layer,” explains Nicoli. “This is the first evidence of this function, which makes us rethink the properties of stem cells.”
Like zebrafish, the skin of the human fetus is organized into two layers: an outer protective layer called the periderm and an underlying layer of stem cells that separates the periderm from the extracellular matrix.
Sumigray, Nicoli and their colleagues compared their findings on the embryonic zebrafish fin fold to a bilayer model of human epidermis. Their modeling revealed that collagen and laminin matrices similarly influence human skin cells. Specifically, they found that laminin inhibited proteins responsible for the formation of desmosomal junctions.
This study supports ongoing research into intra-tissue communication and offers a new approach to strengthening cellular connections within the body’s protective tissues. The findings could open a new avenue for developing methods of skin healing through tissue engineering and regenerative medicines, including organ repair and skin grafts.
“This has broad implications,” says Nicoli. “Although stem cells are present throughout the adult body, they generally remain dormant. It would be exciting if we could one day guide stem cells to create personalized mechanical shields that protect the tissues in which they reside.”
More information:
Helen Mengze He et al, Epidermal stem cells control the repair of peridermal lesions via matrix specialization of intercellular junctions, Natural communications (2025). DOI: 10.1038/s41467-025-64040-7
Provided by Yale School of Medicine
Quote: Unlocking the skin’s natural healing power for regenerative medicine (October 13, 2025) retrieved October 13, 2025 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.