Credit: JACS Au (2024). DOI: 10.1021/jacsau.3c00577
Pathogens hijack host cell functions by expressing or secreting effector proteins, thereby creating environments conducive to their survival and reproduction. These pathogenic microorganisms, including eukaryotic parasites, prokaryotic bacteria, and viruses, express effector proteins that function as their “ammunition depot.”
These proteins are essential for pathogen survival and dissemination because they enhance invasion efficiency, suppress the host immune system, or initiate pathogen replication. For example, viruses can interfere with host signaling pathways, pushing cells into states that favor viral replication.
Similarly, some bacteria secrete toxins that disrupt the cytoskeleton of host cells, facilitating the invasion and spread of pathogens. Additionally, pathogens can evade immune surveillance by suppressing the host immune response, increasing the likelihood of successful infection.
Toxoplasmosis, a systemic disease caused by Toxoplasma gondii, poses particularly high risks to pregnant women and immunocompromised individuals. T. gondii is spread by reaching cats as the definitive host and is transmitted by intermediate hosts, including humans and other warm-blooded animals.
To evade host immune defenses, T. gondii secretes the effector protein TgPDCD5, which induces apoptosis of host macrophages. Although the exact mechanism by which TgPDCD5 enters host cells is not fully understood, studies suggest that it may do so via endocytosis mediated by interaction with heparin or heparan sulfate proteoglycans on the host cell surface. However, as a biological macromolecule, proteins typically face significant challenges in crossing cell membranes.
Professor Chun-Hua Hsu’s research team at National Taiwan University aims to elucidate the mechanism and function of TgPDCD5 through structural biochemical and biophysical research.
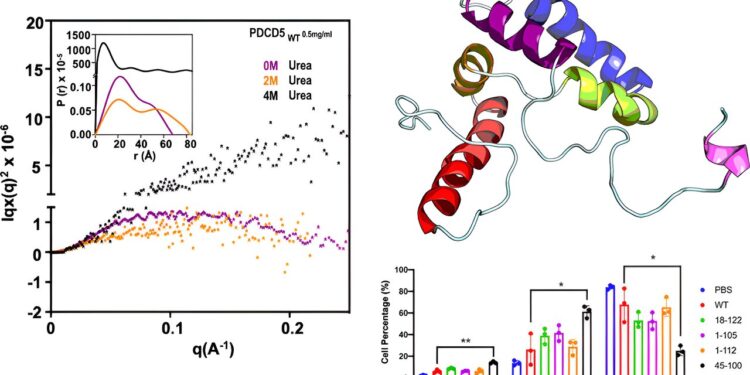
In an article published in JACS AuThe team used techniques such as circular dichroism spectroscopy, fluorescence spectroscopy, and synchrotron small-angle X-ray scattering to provide the first conclusive evidence that TgPDCD5 exhibits the characteristics of a molten globule. Further NMR analysis revealed that TgPDCD5 was a helical bundle with an extended N-terminal helix, also exhibiting molten globular features.
NMR perturbation studies showed that heparin/heparan sulfate binding involves both the heparan sulfate/heparin proteoglycan binding motif and the central region, with the interaction being influenced by proline isomerization of the P107 residue.
Interestingly, the team found that another proline isomerase, TgCyp18, secreted by the T. gondii “ammunition depot,” plays a regulatory role in the cis-trans isomerization of TgPDCD5 proline residues, facilitating the binding and release of TgPDCD5 to and from heparan sulfate-based polysaccharides. This regulation is akin to that of a ship dropping anchor to stabilize itself during docking and weighing anchor to set sail when departing.
Therefore, the research team proposed a molecular mechanism for TgPDCD5 entry into cells: it first binds to heparan polysaccharides on the cell surface and then, due to its molten globule state, maintains sufficient flexibility to perform a subtle “protein dance” that allows membrane translocation into the cell.
More information:
Gloria Meng-Hsuan Lin et al, Proline isomerization and molten globular property of TgPDCD5 secreted by Toxoplasma gondii confer its regulation of heparin sulfate binding, JACS Au (2024). DOI: 10.1021/jacsau.3c00577
Provided by National Taiwan University
Quote:Unveiling the complex and subtle dance of proteins: understanding how parasites disarm host defenses (2024, August 20) retrieved August 20, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.