by IMBA- Institute of Molecular Biotechnology of the Austrian Academy of Sciences
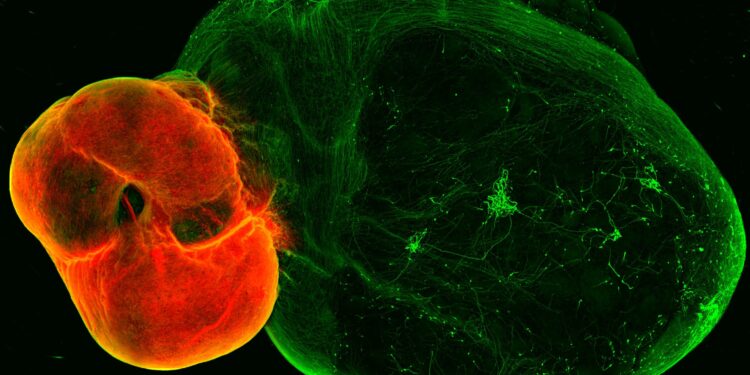
Dopamine neurons in the ventral midbrain (red) and ventral midbrain projections in striatal and cortical tissues (green). Credit: (c) Daniel Reumann/IMBA
A new organoid model of the dopamine system sheds light on its complex functionality and potential implications for Parkinson’s disease. The model, developed by Jürgen Knoblich’s group at the Institute of Molecular Biotechnology (IMBA) of the Austrian Academy of Sciences, reproduces the structure, connectivity and functionality of the dopamine system.
The study, published in Natural methodsalso reveals the lasting effects of chronic cocaine exposure on the dopaminergic circuit, even after withdrawal.
A finished race, the early morning dose of caffeine, the smell of cookies in the oven: these rewarding moments are all due to a dose of dopamine, a neurotransmitter, released by the neurons of a neuronal network in our brain, called “dopamine reward pathway”. “.
As well as mediating the feeling of “reward”, dopamine neurons also play a crucial role in fine motor control, which is lost in diseases such as Parkinson’s. Despite the importance of dopamine, the main characteristics of this system are not yet understood and there is no cure for Parkinson’s disease. In their new study, Jürgen Knoblich’s group at IMBA developed an organoid model of the dopamine system, which recapitulates not only the morphology and nerve projections of the system, but also its functionality.
A model of Parkinson’s disease
Tremors and loss of motor control are characteristic symptoms of Parkinson’s disease and are caused by a loss of neurons that release the neurotransmitter dopamine, called dopamine neurons. When dopamine neurons die, fine motor control is lost and patients develop tremors and uncontrollable movements. Although the loss of dopamine neurons is crucial in the development of Parkinson’s disease, the mechanisms by which this occurs and how we can prevent or even repair the dopamine system are not yet understood.
Animal models of Parkinson’s disease have provided insight into Parkinson’s disease; However, because rodents do not naturally develop Parkinson’s disease, animal studies have been unsatisfactory in recapitulating the characteristic features of the disease. Additionally, the human brain contains many more dopamine neurons, which also wire differently in the human brain, sending projections to the striatum and cortex.
“We sought to develop an in vitro model that recapitulates these human characteristics in so-called brain organoids,” explains Daniel Reumann, a former doctoral student. student in the laboratory of Jürgen Knoblich at IMBA and first author of the article. “Brain organoids are three-dimensional structures derived from human stem cells, which can be used to understand both the development and function of the human brain,” he explains further.
The team first developed organoid models of the ventral midbrain, striatum and cortex – the regions connected by neurons in the dopamine system – then developed a method to fuse these organoids. As is the case in the human brain, dopaminergic neurons in the midbrain organoid send projections to organoids in the striatum and cortex.
“Somewhat surprisingly, we observed a high level of dopamine innervation, as well as synapses forming between dopamine neurons and neurons in the striatum and cortex,” Reumann recalls.
To assess whether these neurons and synapses are functional, the team collaborated with Cedric Bardy’s group at SAHMRI and Flinders University, Australia, to determine whether neurons in this system would begin to form functional neural networks. Indeed, when the researchers stimulated the midbrain, which contains dopamine neurons, neurons in the striatum and cortex responded to the stimulation. “We have successfully modeled the dopamine circuit in vitro, because the cells not only connect correctly, but also work together,” explains Reumann.
The organoid model of the dopamine system could be used to improve cellular therapies against Parkinson’s disease. In early clinical studies, researchers injected dopaminergic neuron precursors into the striatum to try to compensate for the loss of natural innervation.
However, these studies have had mixed success. In collaboration with Malin Parmar’s laboratory at Lund University, Sweden, the team demonstrated that dopamine progenitor cells injected into the dopamine organoid model mature into neurons and extend neuronal projections within the organoid.
“Our organoid system could serve as a platform for testing the conditions of cell therapies, allowing us to observe the behavior of precursor cells in a three-dimensional human environment,” explains Jürgen Knoblich, the corresponding author of the study. “This allows researchers to study how progenitors can be differentiated more efficiently and provides a platform that allows us to study how to recruit dopamine axons to target regions, all at high throughput.”
Reward System Overview
Dopamine neurons also fire whenever we feel rewarded, forming the basis of the “reward pathway” in our brain. But what happens when dopamine signaling is disrupted, such as in addiction? To study this question, the researchers used a well-known dopamine reuptake inhibitor, cocaine. When organoids were chronically exposed to cocaine for 80 days, the dopamine circuit changed functionally, morphologically, and transcriptionally.
These changes persisted even when cocaine exposure was stopped 25 days before the end of the experiment, which simulated withdrawal conditions. “Even almost a month after cocaine exposure stopped, the effects of cocaine on the dopamine circuit were still visible, meaning we can now study what the long-term effects of overstimulation are dopaminergic in a human-specific in vitro system.” Reumann said.
More information:
Jürgen Knoblich et al, In vitro modeling of the human dopaminergic system using spatially arranged ventral midbrain – striatum – cortex assembloids, Natural methods (2023). DOI: 10.1038/s41592-023-02080-x
Provided by IMBA – Institute of Molecular Biotechnology of the Austrian Academy of Sciences
Quote: Uncovering the secrets of the brain’s dopaminergic system (December 5, 2023) retrieved on December 5, 2023 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.