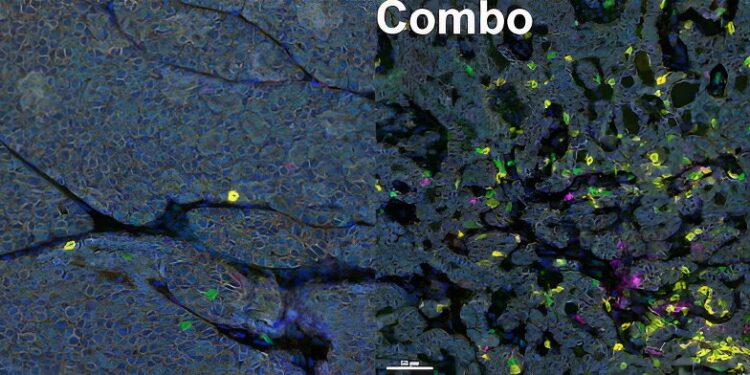
Anti-tumor immune cells in the tumor before (left) and after (right) combination therapy. After combination therapy, many more anti-tumor immune cells (T cells are shown in green and CD8+ T cells in yellow) entered the tumor. Credit: Cell Reports (2024). DOI: 10.1016/j.celrep.2024.114532
A team of researchers from MUSC Hollings Cancer Center has discovered a way in which triple-negative breast cancer (TNBC) cells become resistant to immunotherapy and tested a two-pronged treatment strategy capable of restoring sensitivity to immunotherapy in a preclinical model.
The team, led by Besim Ogretmen, Ph.D., SmartState Chair in Lipidomics and Drug Discovery at MUSC, presents its findings in Cell Reports.
TNBC accounts for 10 to 20 percent of all breast cancer cases and is highly aggressive, with significantly lower five-year survival rates than other breast cancers. It is twice as likely to occur in women under 40 than in those over 50 and is more common in black women. TNBC is often diagnosed late, even after metastasis, making it difficult to treat.
TNBC gets its nickname “triple negative” because patients with the disease already have three odds stacked against them. Because TNBC cells lack two necessary receptors, hormonal therapies, the mainstay of breast cancer treatment, won’t work. Therapies that specifically target the HER2 protein won’t work either, because TNBC patients either lack it or have very low levels of it. These three odds leave patients with TNBC, especially metastatic TNBC, with few treatment options.
“There is no silver bullet for triple-negative breast cancer,” said Wyatt Wofford, MD, a doctoral candidate in MUSC’s Ogretmen Lab and lead author of the paper.
“Typically, response rates for TNBC are about 15 to 20 percent, which leaves about 80 percent of your patients with metastatic TNBC without a good treatment option.”
In recent years, immunotherapy has emerged as a breakthrough therapy for many blood cancers, but its success is limited in solid tumors such as TNBC. The checkpoint inhibitor pembrolizumab has been approved for recurrent TNBC in patients with at least 10% of tumor cells expressing the immunosuppressive protein PD-L1.
PD-L1 on the surface of cancer cells binds to PD-1 on the surface of immune cells called T cells, preventing them from doing their job of destroying cancer and putting the brakes on the immune system. Checkpoint inhibitors release the brakes by preventing PD-1 and PD-L1 from binding, allowing the immune system to resume its attack on the cancer.
The Ogretmen lab studies sphingolipids, fatty molecules that provide rigidity and stability to the cell membrane. One of the sphingolipids is ceramide. Enzymes synthesize a variety of ceramides, each with a different fatty acid chain length and a different role in cancer development and progression.
One of these enzymes, ceramide synthase 4 (CERS4), created ceramides that were important for maintaining membrane stability.
“The ceramide generated by this enzyme appears to be important for keeping the tumor cell membrane intact so everything stays where it needs to be,” Ogretmen said. “When you lose that ceramide, the PD-L1 protein doesn’t stay on the surface of the cells.”
In their study, Ogretmen and Wofford found that when levels of this enzyme dropped and not enough of the ceramides it produced were available, the membrane became unstable, allowing PD-LI to descend into the tumor cell.
There, hidden inside the cell, PD-L1 was not exposed to the immunotherapeutic agent as it was on the surface. With this discovery, Ogretmen and Wofford identified a mechanism by which TNBC becomes resistant to immunotherapy. They also showed that internalized PD-L1 could promote cellular pathways linked to metastasis.
The MUSC team then wanted to know if it was possible to make tumor cells vulnerable to immunotherapy again. The researchers treated a mouse model of TNBC lacking the CERS4 enzyme with a PD-L1 inhibitor and an existing anticancer drug that blocks one of the metastatic cell pathways promoted by internalized PD-L1.
Before treatment, mice uniformly developed lung metastases. However, after treatment, the tumor cell membrane regained stability and PD-L1 remained on the cell surface where it was exposed to immunotherapy and unable to promote tumor growth and metastasis.
Ogretmen and Wofford turned to Ozgur Sahin, Ph.D., SmartState chair in the department of biochemistry and molecular biology, to lead the drug studies. Wofford still remembers his excitement when he first saw the results of those studies.
“When we looked at tumor growth over time, using either immunotherapy alone or the metastatic pathway inhibitor alone had virtually no effect. It was almost like giving the cancer cells water – they just weren’t listening,” he said. “And then when you combined those two together, there was a really pronounced response. The tumors stopped growing and even started to shrink.”
For Ogretmen, the discovery of a biological mechanism of resistance in TNBC, while interesting in its own right, is exciting in large part because it will allow researchers to manipulate that mechanism to restore sensitivity to treatment.
“In this study, we were looking not only to study how TNBC becomes more resistant to immunotherapy, but also to use what we learned to make these cancer cells more responsive to immunotherapy,” he said.
Currently, it is not possible to move to the clinic with the pathway inhibitor tested in the study. The team’s next steps are to identify other compounds, ideally existing and approved drugs that act on the same metastatic pathway and that could be used in a combination therapy with a PD-L1 inhibitor in patients with TNBC.
“We have some exciting new leads and results,” Ogretmen said. “We are making progress in finding more viable combinations that we can actually apply in the clinic.”
More information:
Wyatt Wofford et al, Alterations in ceramide synthesis induce PD-L1 internalization and signaling to regulate tumor metastasis and response to immunotherapy, Cell Reports (2024). DOI: 10.1016/j.celrep.2024.114532
Provided by the Medical University of South Carolina
Quote: Double Trouble for Triple-Negative Breast Cancer: Two-Pronged Strategy Restores Immunotherapy Sensitivity (2024, September 9) Retrieved September 9, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.