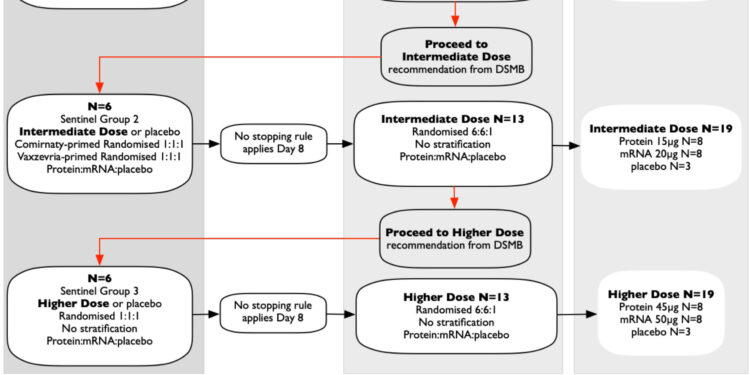
Test profile. Credit: eBioMedicine (2023). DOI: 10.1016/j.ebiom.2023.104878
Two Melbourne-made COVID-19 vaccines have shown strong potential to provide an improved approach to boosting immunity against SARS-CoV-2 variants, according to interim results from a Phase 1 clinical trial.
Published in eBioMedicine76 healthy adults from Melbourne, aged 18 to 64 years, previously vaccinated with licensed SARS-CoV-2 vaccines, were randomized to receive a fourth dose of a novel protein vaccine, an mRNA vaccine or ‘a placebo.
Both vaccines demonstrated strong boosting capabilities in a highly immunized population and an immune response of remarkable magnitude, including against omicron subvariants. Additionally, no safety signals were observed in either candidate.
The two vaccine candidates, created by researchers at the Peter Doherty Institute for Infection and Immunity (Doherty Institute) and the Monash Institute of Pharmaceutical Sciences (MIPS), differ from most existing vaccines used in the world because they concentrate the immune system. response to the end of the SARS-CoV-2 spike protein, called the receptor binding domain (RBD). The RBD allows the virus to enter and infect cells in the body and produce more than 90% of neutralizing antibodies (antibodies that can block the virus) after infection with SARS-CoV-2.
The two candidates are:
- RBD protein vaccine: uses an artificial part of the viral protein, rather than genetic material or another virus, to elicit an immune response.
- RBD mRNA Vaccine: Represents the viral genetic sequence of mRNA that codes for the spike, which leads to the production of the RBD protein in the recipient.
Professor Terry Nolan of the University of Melbourne, head of the Vaccines and Immunization Research Group at the Doherty Institute, who led the first phase 1 trial in humans, said the team was exceptionally happy with the results.
“Post-marketing studies of omicron-directed whole-spike bivalent mRNA booster vaccines have shown a modest increase in immune responses to omicron variants compared to ancestral vaccine boosters,” Professor Nolan said.
“Because our two vaccines focus the immune response on the receptor binding domain, they avoid unnecessary immune responses against other parts of the spike protein and therefore could provide a more effective approach to boosting immunity against the virus. , thus presenting a strong case for moving to phase 2.2 clinical trials.”
Professor Colin Pouton of MIPS, who led the development of the RBD mRNA vaccine, said the mRNA vaccine showed a strong immune response even at the lowest dose tested.
“So far, preclinical and clinical studies have shown that our RBD mRNA vaccine provides a powerful boost at low doses, suggesting the very real potential to develop a multivalent vaccine, on an annual basis, and protect against new emerging variants of COVID-19. , which would be at the origin of the “waves” that we still know,” said Professor Pouton.
“New strategies are still needed to improve the effectiveness of vaccines against COVID-19 variants and reduce mortality rates, particularly among older and vulnerable patients. In the case of our mRNA vaccine, we also saw early potential to address the immune imprinting issue. , which should also be an essential element for a new generation vaccine. »
The team is currently exploring options to advance these vaccines into Phase 2 trials.
More information:
Terry M. Nolan et al, Interim results from a randomized, placebo-controlled phase I trial of novel SARS-CoV-2 beta variant receptor-binding domain mRNA and recombinant protein vaccines, such as 4th dose booster, eBioMedicine (2023). DOI: 10.1016/j.ebiom.2023.104878
Provided by the Peter Doherty Institute for Infection and Immunity
Quote: Two COVID-19 vaccines show strong boosting potential in clinical trial (December 12, 2023) retrieved December 12, 2023 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.