Credit: Structure (2024). DOI: 10.1016/j.str.2024.08.002
A toxic version of a certain protein may differentially affect brain, spinal cord and skeletal muscle tissues, leading to the complex development and progression of amyotrophic lateral sclerosis (ALS), according to a new team-led study. of researchers from the Penn State College of Medicine. .
The study represents a step forward in understanding the physiological processes that may give rise to ALS and identifies a potential therapeutic target for future ALS treatments. The team published their findings in the journal Structure.
“In ALS, as in other neurodegenerative diseases, certain proteins tend to aggregate into harmful groups. Superoxide dismutase 1, or SOD1, is associated with ALS,” said lead author Nikolay Dokholyan, G .Thomas. Passananti Professor at Penn State College of Medicine and professor of biochemistry and molecular biology.
Dokholyan explained that SOD1 typically exists as a dimer, a protein made up of two identical units. Under certain conditions, SOD1 will change shape and reassemble into a three-unit form called a trimer. “We need to understand how SOD1 trimers kill cells and the mechanisms involved,” he said.
ALS is a progressive neurodegenerative disease that affects nerve cells, called neurons, in the central nervous system and leads to muscle weakness and atrophy. SOD1 mutations have been implicated in approximately 20% of ALS cases with a known genetic cause and in a small percentage of cases with no known genetic link.
Previous research has shown that SOD1 trimers appear to acquire a toxic function compared to dimers. SOD1 trimers are associated with increased cell death in ALS models, but the exact underlying molecular mechanism is not known, Dokholyan said.
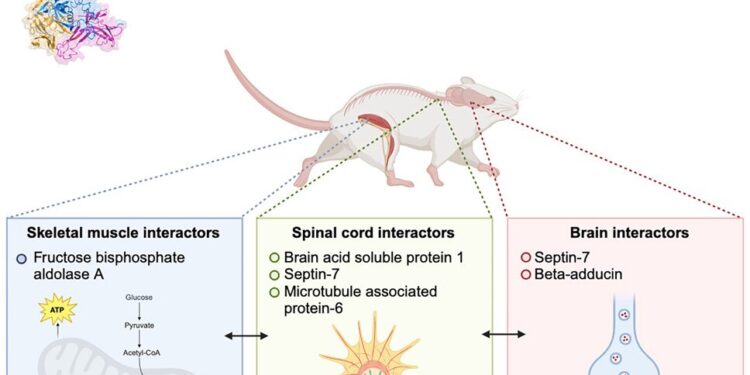
To study the role that SOD1 trimers play in cellular dysfunction and degeneration, the team examined which proteins bind to SOD1 trimers.
Dokholyan explained that they introduced the SOD1 trimers into three different types of mouse tissue (brain, spinal cord, and muscle tissue) and observed which proteins attached to the trimers. They then compared the protein binding partners of SOD1 trimers in the three tissues with the binding partners of SOD1 dimers.
“We were trying to see if there were any new proteins that would interact with this toxic protein that had never been observed before,” said Brianna Hnath, a doctoral student in biomedical engineering at Penn State and co-author of the study. “The goal was to find potential pathways by which this SOD1 trimer could have a toxic pathway.”
Brianna Hnath, left, is a doctoral student in biomedical engineering at Penn State and co-author of the study. Credit: Dokholyan laboratory / Penn State
The researchers found that SOD1 trimers interact with different proteins depending on tissue type, which they believe may partly explain the complex and multifaceted nature of ALS.
In brain and spinal cord tissues, SOD1 trimers bind to proteins involved in maintaining structure, function, and communication between nerve cells. The team also discovered that SOD1 trimers activate pathways related to cellular aging, which may contribute to neuronal dysfunction and degeneration.
In muscle tissue, SOD1 trimers bind to proteins involved in metabolic processes. As a result, this interaction can directly interfere with metabolism and energy production within muscle cells.
“The fact that we found different impacts in the three tissue types, instead of a uniform impact, means that there could be different mechanisms leading to cell dysfunction and death, depending on the cell type ” said Hnath.
This finding challenges the traditional notion that muscle wasting in ALS is a secondary result of motor neuron degeneration: when these neurons are not functioning normally, muscle cells are not stimulated, which can lead to muscle atrophy. , explained Dokholyan.
However, the study suggests that there may also be processes within muscle cells that are disrupted by SOD1 trimers, potentially causing muscle cell dysfunction and death, thereby contributing to muscle wasting and death. neurons.
“Both neurons and muscle cells are affected,” Dokholyan said. “On the neuron side, it potentially affects the ability of neurons to connect to muscles, while on the muscle side, it affects metabolism.”
In particular, the study identified the septin-7 protein as a binding partner for SOD1 trimers but not for native SOD1 dimers. Septin-7 plays a role in essential nerve cell processes, such as maintaining cellular structure and communication, and has been linked to ALS in previous studies. Binding with SOD1 can disrupt these functions, leading to neuron degeneration.
This raises the question of whether treating this interaction could slow or disrupt the progression of ALS, making septin-7 a potential therapeutic target, Dokholyan said.
He noted that additional research is needed to better understand the potential role of SOD1 trimers in the development of ALS, how they may lead to cell dysfunction and death, as well as the specific role of septin-7, which could guide future development of potential therapies. .
More information:
Esther Sue Choi et al, Unveiling the double-edged sword: SOD1 trimers possess tissue-selective toxicity and bind septin-7 in motor neuron-like cells, Structure (2024). DOI: 10.1016/j.str.2024.08.002
Provided by Pennsylvania State University
Quote: Toxic protein may contribute to the development of amyotrophic lateral sclerosis (October 14, 2024) retrieved October 14, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.