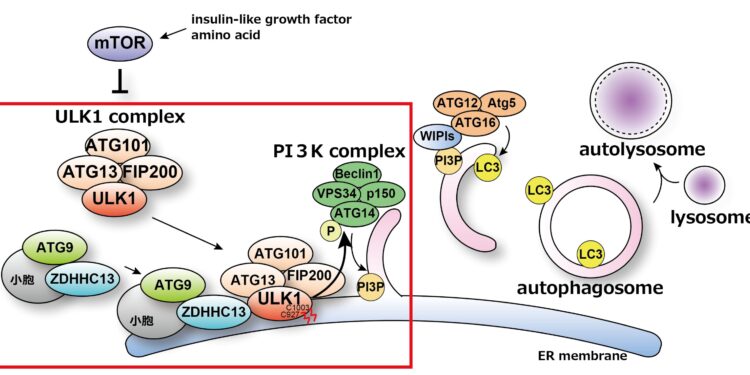
Upon induction of autophagy, ZDHHC13 is recruited to autophagosome formation sites along with ATG9A. ULK1 is palmitoylated at Cys927 and Cys1003 ZDHHC13 residues and the ULK1 complex are anchored at an autophagosome formation site. ULK1 palmitoylation promotes ATG14L phosphorylation that leads to VPS34 activation. The activated PI3-kinase complex produces PI3P at autophagosome formation sites. These sequential reactions trigger efficient induction of autophagy. Credit: Keisuke Tabata
An international research team led by Osaka University has identified a new mechanism crucial for triggering autophagy, a self-degradation process used by cells to eliminate unnecessary or damaged components. In recent years, autophagy has also been recognized for its role in regulating aging and lifespan.
During autophagy, intracellular molecules and structures are sequestered in a membrane structure called autophagosome, which is then degraded in lysosomes. It is well established that autophagosome formation involves the coordinated action of several autophagy-related proteins.
The researchers have already shown that autophagosome formation takes place on the endoplasmic reticulum membrane, in close proximity to the mitochondria of cells. They have also discovered that the PI3K complex, a protein linked to autophagy, is essential for this formation process. The activity of the PI3K complex is controlled by the ULK1 complex.
The ULK1 complex is known to translocate from the cytoplasm to the endoplasmic reticulum membrane, where autophagosomes are formed at the onset of autophagy. However, the underlying mechanism and significance of this process were not fully understood until now.
In an article published in Nature CommunicationsThe research team revealed that palmitoylation of ULK1 triggers a series of reactions that initiate autophagy.
The team identified ZDHHC13, a palmitoylation enzyme, as a key player in this process through their search for intracellular factors involved in the initiation of autophagy.
Mutations in the ZDHHC13 gene have been linked to various diseases, including Huntington’s disease, while autophagy is also involved in the development and progression of diseases such as cancer and neurodegenerative disorders.
Lead author Maho Hamasaki explained, “Our team discovered that ZDHHC13 palmitoylates ULK1, thereby localizing the ULK1 complex to sites of autophagosome formation. This palmitoylation is also involved in the phosphorylation of the ATG14L protein within the PI3K complex, which in turn regulates the activity of the PI3K complex.”
Understanding the molecular mechanisms that initiate autophagy should improve our knowledge of autophagy-related diseases.
Lead author Keisuke Tabata added: “Autophagy not only provides a source of nutrients through intracellular degradation, but also plays a crucial role in maintaining normal cellular function and preventing various pathologies. We will continue to study how autophagy begins, thereby deepening our understanding of the mechanisms underlying the associated diseases.”
More information:
Keisuke Tabata et al, Palmitoylation of ULK1 by ZDHHC13 plays a crucial role in autophagy, Nature Communications (2024). DOI: 10.1038/s41467-024-51402-w
Provided by Osaka University
Quote: The triggering mechanism of autophagy revealed (2024, September 24) retrieved September 24, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.