Proposed mechanistic model, showing how hydroxide movement is limited by slow exchange with blocking acetate ions. Credit: De Toh et al, Natural energy (2023). DOI: 10.1038/s41560-023-01404-7
Bipolar membranes (BPM) are a class of ion exchange membranes typically comprising a cation exchange layer and an anion exchange layer. Although these membranes have recently been integrated into various electrochemical devices for a wide range of applications, the processes underlying their operation are not yet fully understood.
Researchers at the Massachusetts Institute of Technology (MIT) recently developed a new mechanistic model that explains the forward polarization mechanisms of BPMs in mixed electrolytes with varying acidities and basicities. Their model, introduced in Natural energycould guide the development of strategies to overcome the problem of ionic blockages, which can harm the performance of forward-biased BPM devices.
“We were initially trying to design an electrolyzer that converts carbon dioxide CO2 into useful feedstocks or fuels using bipolar membranes (BPM),” Yogesh Surendranath, co-author of the paper, told Tech Xplore. “To provide some context, CO2 Electrolyzers are most effective when operating with alkaline electrolyte solutions such as potassium hydroxide, but because CO2 is an acidic gas, it reacts with alkaline solutions to produce carbonate solutions over time.
The chemical reactions that can occur when CO2 electrolyzers operated with alkaline solutions have a significant impact on the efficiency of these technologies, thus preventing their widespread and long-term adoption. In fact, these reactions cause CO2 the raw material migrates and escapes into a different part of the electrolyzer, while making the electrolyte solutions used less alkaline.
“We imagined that with forward-biased BPMs, we could design an electrolyzer that would take the carbon electrolyte and react it in an acid-base reaction in the BPM to reform CO.2 gas,” Surendranath explained. “This in turn would allow us to build a stable and viable electrolyzer in the long term.”
While working on the electrolyzer they envisioned, Surendranath and his colleagues realized that the electrochemistry of forward-biased BPMs was still poorly understood. They therefore undertook to fill certain gaps in the literature, which would ultimately allow them to create their electrolyzer.
“Once we conducted some preliminary experiments to shed light on how direct bias BPMs work, we realized we had the opportunity to undertake a much more interesting study to uncover the mechanism of direct bias BPMs, and decided to dive headfirst into this effort,” Wei Lun Toh, co-author of the paper, told Tech Xplore.
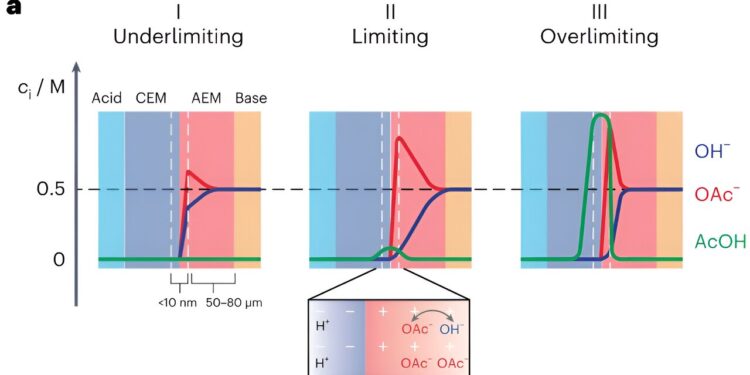
A BPM essentially consists of a positively charged membrane that transports anions (i.e. the anion exchange membrane) and a negatively charged membrane that transports cations (i.e. the cation exchange membrane). Forward polarization BPMs are BPMs in which ions are transported from external solutions to the so-called bipolar junction.
“In forward polarization, the electrolyzer is polarized in such a way that acidic cations can enter the cation exchange membrane and basic anions can enter the anion exchange membrane,” Toh explained. “These acidic and basic ions can then meet at the interface between the two membranes and undergo a neutralization reaction. For example, hydrogen ions and hydroxide ions meet to form water.”
Consequences of carbonate accumulation in BPM carbon dioxide electrolyzers. Credit: De Toh et al, Natural energy (2023). DOI: 10.1038/s41560-023-01404-7
The interface between cation exchange and anion exchange membranes is also known to be associated with a voltage drop (i.e. membrane voltage). Notably, recent experimental studies have shown that this membrane tension is linked to the degree of acidity or base of the interface.
“Membrane tension therefore controls which acids and bases can react in the BPM,” Toh said. “If you have a mixture of a weak basic anion and a strong basic anion in one of the solutions, for example acetate and hydroxide, then these two anions can both penetrate the exchange membrane anions.
“However, depending on the membrane voltage, only the hydroxide, which is the most reactive ion, can react. In this case, the acetate ion would still be present in the anion exchange membrane, but would eventually by blocking the movement of the hydroxide, because it takes up space in the membrane.
The hindered movement of some ions due to the blocking of other ions is known as “ion blocking”. Ionic blockages can significantly limit the current that can flow through an electrolyzer without resulting in significantly higher voltages.
“In a sense, the way these ions move around each other is like moving on a sidewalk: they share the same ‘path,’ so to speak, and the path only allows one ion to pass through. So at some point the ions need to do a little dance and move in concert to allow the current to flow,” Toh said.
“For example, the hydroxide and acetate swap places so that the hydroxide can move toward the center of the BPM and the acetate can move out of its way and away from the center. The real picture How ions move is much more complicated, but this analogy of moving sidewalks is a good heuristic to start thinking about the movement of ions in BPMs.”
In their paper, Surendranath and colleagues describe a new model that better explains how the efficiency with which forward-biased BPMs harvest energy is affected by ionic blockages. Although the researchers explained their model using acetate solutions as an example, it also applies to other solutions that can block the movement of hydroxides, such as carbonate,
The ion blocking phenomenon described by Surendranath and colleagues has a direct impact on CO functioning2 electrolyzers. In fact, increased concentrations of acetate or carbonate are known to cause serious increases in cellular overpotentials.
“Above all, we hope that our paper will help highlight not only the challenges, but also the promises of BPMs, and bring them to the forefront of energy research,” Surendranath said. “Direct bias BPM devices have already been demonstrated in numerous academic studies, including as CO2 electrolyzers, but often they are not recognized as such, and thus opportunities to understand how to better optimize the energy efficiency of these devices are missed.
In the future, the model introduced by this team of researchers could inform the development of new strategies to overcome the problem of ionic blockages in BPMs, which generalize well across all forward-biased BPM devices. Surendranath and his colleagues hope this will help further refine BPMs and improve the performance of electrolyzers, making them easier to implement on a large scale.
“There are still aspects of how BPM works that remain unclear to us, we continue to progress in our understanding,” added Surendranath. “In particular, we are interested in the molecular details of how the interface at the center of BPM works and hope to be able to bring a new perspective to it as inorganic chemists.”
More information:
Wei Lun Toh et al, The role of ionic blockades in controlling the efficiency of energy harvesting in forward-biased bipolar membranes, Natural energy (2023). DOI: 10.1038/s41560-023-01404-7
© 2024 Science X Network
Quote: The model describes how ionic blockages influence energy harvesting in forward-biased bipolar membranes (January 15, 2024) retrieved January 15, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.