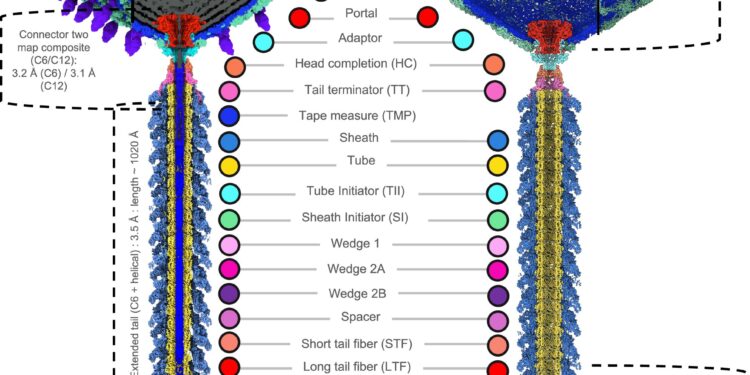
Complete composite reconstruction and bacteriophagus model φte. Credit: Nature communications (2025). DOI: 10.1038 / S41467-025-58514-X
Plants are sensitive to a wide range of pathogens. For the common potato plant, one of these threats is the pectobacterium atrosepticum, a bacteria which causes stems to be blackened, the tissues decompose and often leads to the death of plants, causing significant agricultural losses each year.
In 2012, researchers isolated a new virus that infects and kills this bacteria – a bacteriophagus called φte (sentence). Now, for the first time, scientists discovered the atomic structure of φte, revealing a possible infection mechanism which can be more complex than we thought before.
The study, published earlier this month in Nature communicationsis the result of a multidisciplinary collaboration between researchers from the Okinawa Institute of Sciences and Technology (OIST) and the University of Otago. It brings together expertise in several areas, including virology, structural biology, molecular genetics, protein engineering, biochemistry and biophysics.
Belonging to one of the most omnipresent viral classes, φte is a bacteriophagus that particularly interests the community of molecular genetics. In addition to its role in the fight against pathogens of plants, it serves as a model virus commonly used in research to study how bacteriophages interact with their host bacteria.
Using cryo-electron microscopy (Cryo-EM), the team managed to capture the complete virion φte for atomic resolution. They discovered that the virus has a unique topology compared to other viruses from the same family.
These new ideas have helped to understand the conformational changes that allow the virus to release its DNA in the host and to initiate an infection. φte has also proven to have a relatively larger capsid, capable of adapting to a larger genome.
In addition, the team has identified structural elements that seem to play an essential role in maintaining the stability and the function of the virion. Direct visualization allowed them to resolve a protein known as the measuring ribbon protein (TMP), which is important for assembly and the function of the virion.
By comparing φte to related viruses, they have identified several shared characteristics as well as notable differences. Based on these results, the researchers proposed a model describing how φte could initiate its attack on the host.
This research advances our understanding of bacteriophages, such as φte, and could have important implications. It allows better for scientists to conceive organic agents that can fight against a range of bacterial plant diseases. These agents serve as precious alternatives to traditional chemical treatments and antibiotics.
More information:
James Hodgkinson-Bean et al, Global Structural Survey of the Flagelotrope myophagus φte infecting the agricultural pathogen pectobacterium atrosepticum, Nature communications (2025). DOI: 10.1038 / S41467-025-58514-X
Provided by Okinawa Institute of Science and Technology
Quote: The first atomic map of the pathogen of the potato reveals a potential infection mechanism (2025, April 28) recovered on April 28, 2025 from
This document is subject to copyright. In addition to any fair program for private or research purposes, no part can be reproduced without written authorization. The content is provided only for information purposes.