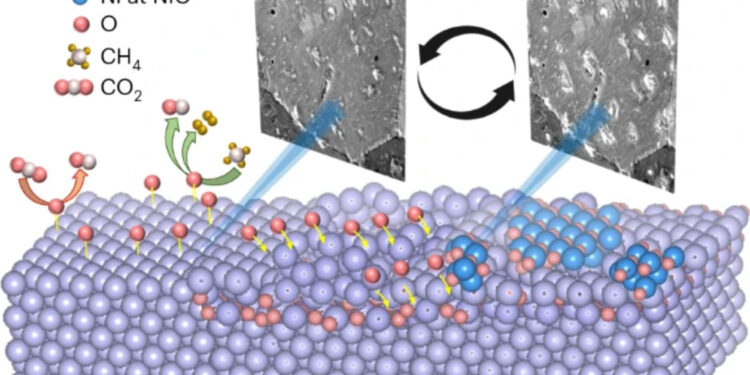
Heterogeneous catalysts are often dynamic in operation. Now the mechanism of CH4 dry reforming on Ni is studied by in situ microscopy and spectroscopy, revealing the formation of metastable surface nickel-oxygen structures from CO2 dissociation which exhibit different catalytic properties and induce rate oscillations. Credit: FHI
Catalysis is one of the key technologies in the chemical industry and has a significant impact on various aspects of our daily lives, including the manufacturing of plastics, the synthesis of drugs, and the production of fertilizers and fuels. It is estimated that more than 90% of chemicals today are manufactured with catalysis in at least one step. Catalysis is a complex process that relies on the precise structural control of several elements at the crossroads of phase (in)stabilities.
While long-term stable catalysts are essential to promote efficient and effective reactions, the reactants undergo major chemical modifications, leading to the formation of the desired end products. In heterogeneous catalysis, the catalyst and reactants exist in different phases.
Among different heterogeneous catalytic processes, dry methane reforming (DRM) has recently received academic attention, because it converts two greenhouse gases, methane (CH4) and carbon dioxide (CO2), to hydrogen (H2) and carbon monoxide (CO). This mixture is also known as syngas and can be used to reduce reliance on fossil fuels by consecutively accumulating larger hydrocarbons via Fischer-Tropsch chemistry.
Although nickel and cobalt catalysts, inexpensive and highly available on Earth, have shown promising activity for DRM, the design of high-performance catalysts is often a challenge because the link between chemical dynamics, formation Surface active species and their reaction pathways are generally absent. This knowledge can only be acquired from so-called operando experiments in which structure and function are probed simultaneously.
A collaborative effort of scientists from the Departments of Inorganic Chemistry and Theory at the Fritz Haber Institute of the Max Planck Society in Berlin has provided fundamental insights into the processes occurring on the catalyst surface and how these modulate catalytic performance during DRM.
The study is published in the journal Natural catalysis.
In particular, the team investigated the role of different oxygen species on a nickel catalyst during DRM using a combination of experimental and computational science techniques, including operando scanning electron microscopy, X-ray photoelectron spectroscopy pressure close to ambient pressure and computer vision.
They highlighted the critical role of dissociative CO2 adsorption in regulating the oxygen content of the catalyst and CH4 Activation. Additionally, they discovered the presence of three metastable oxygen species at the catalyst: surface atomic oxygen, subsurface oxygen, and bulk NiO.X. It is interesting to note that these exhibited different catalytic properties, and that their interaction and transformation gave rise to oscillations in the surface states and in the catalytic function.
They observed that some of the surface oxygen infiltrated into the catalyst mass, thereby reducing the availability of the catalyst for CH.4 activation and promoting CO2 and O diffusion instead.
The extent of the leak was further proven by X-ray spectroscopy and transmission electron microscopy, revealing the presence of oxygen several nanometers below the surface of the catalysts. Therefore, new metal sites were exposed, thereby leading to an increase in the oxygen uptake rate and a decrease in the H concentration.2Product/CO ratio.
Finally, they understood that the co-supply of CO2 is essential for CH4 conversion, probably contributing to its activation as well as the presence of oxygen species.
“It was impressive to see how the metastability of the Ni-O system automatically adjusts the catalytic performance and that one element of the reactants can direct the whole process, which depends on its location and chemistry. We hope that our results can provide further impetus in fine-tuning the longevity and selectivity of catalysis,” says Dr. Thomas Lunkenbein, project leader and co-author of the study.
Understanding the metastability of catalyst surfaces, as well as how to control them to stabilize the dynamic active state, has important implications for the future of catalysis. In particular, it provides information that can be transferred to the industrial level and reactor design where an active state with minimal energy trade-offs is favored.
This could be achieved either by using stronger oxidants, such as water (H2O) and nitrous oxide (N2O), or by working to reduce oxygen leaks in the massif using nanoparticle or thin film technology. The development of catalysts based on tailor-made thin films is the focus of CatLab, a joint research platform between the FHI and the Helmholtz Center Berlin (HZB).
More information:
Luis Sandoval-Diaz et al, Metastable nickel-oxygen species modulate rate oscillations during dry methane reforming, Natural catalysis (2024). DOI: 10.1038/s41929-023-01090-4
Provided by the Max Planck Society
Quote: The advantages and disadvantages of oxygen as a mediator of nickel catalyst performance in dry methane reforming (January 17, 2024) retrieved January 17, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.