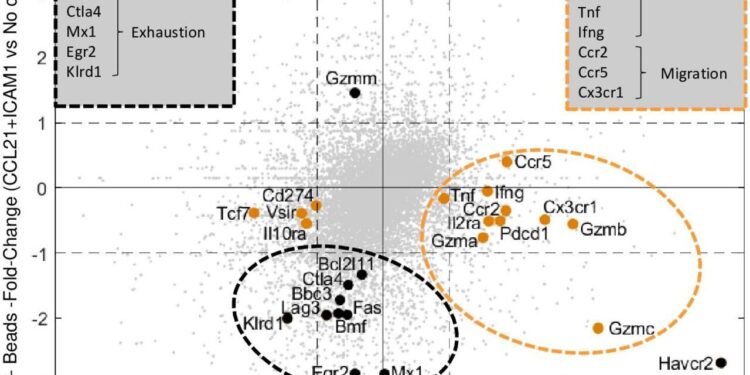
Comparison of significant differences in gene expression profiles between DC-activated cells (orange dots) and bead-activated cells (black dots) on their “peak days” (days 4 and 7, respectively). Credit: Journal of Cancer Immunotherapy (2024). DOI: 10.1136/jitc-2024-009011
Cellular immunotherapy, a major form of cancer treatment, uses the “warriors” of our immune system, T cells, in the war against cancer. In preparation for treatment, doctors take a sample of the patient’s T cells and activate them to divide rapidly and form a massive army of cancer-killing cells that are then reinfused into the patient.
Although using the immune system to fight cancer has considerable potential, the success rate of these treatments has so far been limited. One reason is that after weeks of accelerated division, the “warrior T cells” may be numerous, but they often become exhausted and their destructive power diminishes.
Researchers in the laboratory of Professor Benny Geiger in the Department of Immunology and Regenerative Biology at the Weizmann Institute of Science have developed a new approach that induces an increase in T cell proliferation while maintaining or even enhancing the cells’ destructive capacity.
Geiger began this research about 10 years ago, with his colleague Professor Nir Friedman, who passed away in 2021. In collaboration with Dr Shimrit Adutler-Lieber, they created a “synthetic immune niche” – an artificial molecular environment composed of two proteins that they had carefully selected based on the natural immune system – that allowed cancer cells growing in this environment to reproduce more quickly while maintaining and even improving their destructive abilities.
To promote the application of their findings for potential use in medical practice, Geiger and Friedman continued to study the molecular mechanisms responsible for the unique properties of their synthetic immune niche.
In a study recently published in the Journal of Cancer ImmunotherapyThe researchers looked at two different methods of activating T cells – with and without the presence of the synthetic immune niche – and made findings that could have important ramifications for the future of cellular immunotherapy.
Also participating in the study, led by Dr. Sofi Yado of Geiger’s group, were Rawan Zoabi (also of Geiger’s group), Dr. Shlomit Reich-Zeliger of Friedman’s group, and Dr. Bareket Dassa of Weizmann’s Life Sciences Basic Facilities Department.
“Nir and I approached this project based on our shared interest in the influence of the tumor environment on cellular activity,” Geiger says. “Nir’s contribution was enormous, particularly in developing and applying computational methods and models to track the behavior of individual T cells, while my lab had gained extensive experience in characterizing the interaction between living cells and their environment.”
“When we started, we were trying to find the right ‘recipe’ for the immune niche, and we decided to look for a specific combination of natural immune system proteins that, when introduced into the synthetic niche, would enhance T cell performance and potentially enhance the efficacy of cellular immunotherapy.
“In our early research, we managed to develop such a niche, but the molecular mechanisms it triggered within immune cells remained a mystery.
“The current study focuses on the processes that occur in T cells after they encounter the synthetic niche. It provides insight into the key molecular processes that regulate the balance between the proliferation of killer T cells and their ability to effectively kill targeted cancer cells.”
Killer T cells protect the body by scanning “cellular ID cards,” proteins that appear on cell membranes, which allow them to identify external invaders or internal enemies such as cancer cells and kill them. To be niche-responsive, T cells must first be activated.
In this new study, the scientists compared “specific” T-cell activation, achieved by exposure to a protein derived from the surface of cancer cells, with “nonspecific” T-cell activation, achieved by antibodies that bind to receptors on these cells.
The researchers found that the T cell division rate of mice that had undergone non-specific activation was significantly lower than that of cells that had undergone specific activation. The immune niche improved the situation, so that at the peak of the post-activation phase, the T cell population increased three to five times compared to T cells that had not undergone similar niche treatment.
It was therefore evident that the immune niche did indeed contribute to a higher expansion rate, but the effect of niche stimulation on the killing power of the cells remained unclear.
In studying this question, the scientists noted that each activation method had a different functional time window during which T cells exposed to the immune niche proliferated more rapidly and retained high levels of lethality. These time windows could be important in selecting the optimal time to harvest cells used in cancer treatment.
To get a quantitative measure of the cells’ destructive power, the researchers documented the battle between the immune system’s “warriors” and cancer cells using time-lapse videos created with a microscope at specific intervals.
They noticed that during the first stage, the killing power of cells that were activated nonspecifically and proliferated more slowly was greater than that of cells that had been activated specifically, a finding that suggested an inverse relationship between the rapid division of T cells and their ability to effectively kill cancer cells.
However, four days after stimulation, the synthetic immune niche began to have the opposite effect on cells activated by both methods. Specifically activated cells—which tend to lose their killing power about four days after activation, due to exhaustion attributed to their rapid division rate—emerged from the niche with their killing capacity intact.
The scientists therefore decided that for specifically activated T cells, the fourth day is the optimal window, during which the cells not only divide rapidly but also maintain their high destructive power.
In contrast, in the first days after activation, nonspecifically activated cells tended to divide slowly, while retaining a high killing capacity; the niche, however, encouraged them to divide much more rapidly, resulting in a temporary loss of their killing capacity.
Surprisingly enough, the optimal window for these niche-treated cells was seven days after initial activation, i.e. when their proliferation was maximal and their killing power was fully restored, after being temporarily suppressed during the rapid division phase. This means that T cell yield and killing capacity were particularly high on day seven.
Next, the researchers explored the molecular mechanisms by which the immune niche affected the interaction between the division rate and killing capacity of cells during different optimal time windows.
One of their findings was that throughout the “suppression period,” from days four to six, cells stimulated by the immune niche unexpectedly retained high levels of cellular components associated with the killing mechanism, suggesting that the killing system was still there, albeit turned off.
Only on day 7, when cell yield was maximal, did a sharp decline in the expression of cell exhaustion components occur and cell killing capacity was restored. Specifically activated cells, in contrast, showed a prolonged prominence of cellular components associated with the killing mechanism during their effective time window of day 4.
The Weizmann team filed a patent on the synthetic immune niche, which has so far been tested primarily in mice. It then launched collaborative studies with researchers at Israeli hospitals and the medical industry, hoping to explore a similar system for human cells.
In recent months, using data collected in their follow-up studies, they launched a collaboration with MD Anderson Cancer Center in Houston, Texas, to explore the feasibility of using the system in treating patients.
“Cellular immunotherapy-based treatment has shown very promising results and holds great potential for fighting cancer,” says Geiger. “But its large-scale applicability and efficacy still need to be strengthened, partly because of the need to find the right balance between the number of cells available for treatment and their destructive power.”
“The immune niche we have developed can significantly improve both aspects. If it proves effective in improving cancer immunotherapy in humans, it could offer new horizons for patients who currently have no effective therapeutic options.”
More information:
Sofi Yado et al, Molecular mechanisms underlying modulation of T cell proliferation and cytotoxicity by immobilized CCL21 and ICAM1, Journal of Cancer Immunotherapy (2024). DOI: 10.1136/jitc-2024-009011
Provided by the Weizmann Institute of Science
Quote:’Synthetic immune niche’ approach enhances T cell proliferation without compromising cancer-killing ability (2024, September 9) retrieved September 9, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.