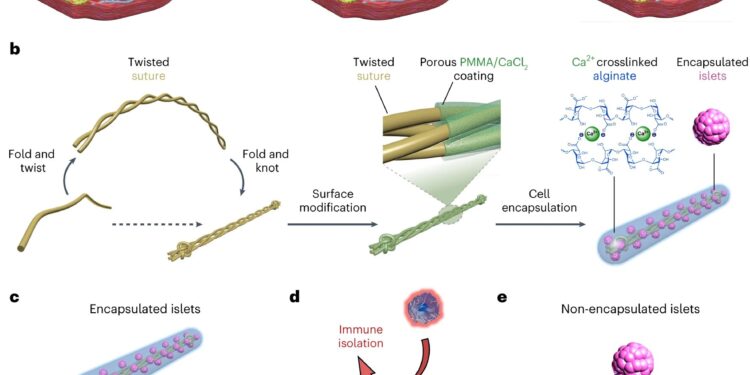
Design of the GAIN system. A, Diagram illustrating the creation of the vascularized subcutaneous site obtained by implantation of a catheter 4 to 6 weeks before transplantation. Removal of the catheter created a vascularized pocket that can be used for implantation of islet encapsulation devices. bDiagram illustrating the manufacture of the island encapsulation device. vs,dSchematic illustrating the concept of the immunoinsulating cellular encapsulation system (vs). The alginate matrix (blue) protects cells from immune interference while allowing the free passage of glucose and insulin necessary for therapeutic function (d). e, Illustration of unencapsulated islets within the vascularized site as an alternative delivery mechanism, suitable only for syngeneic transplants or allogeneic transplants with immunosuppression. Credit: Natural biomedical engineering (2023). DOI: 10.1038/s41551-023-01145-8.
A collaboration between researchers at Cornell and the University of Alberta in Edmonton has created a new technique to treat type 1 diabetes: implanting a device in a pocket under the skin that can secrete insulin while preventing immunosuppression which generally prevents disease management.
This approach would offer a simpler, long-term and less invasive alternative to traditional insulin injections or transplants requiring immunosuppression.
The group’s article is published in Natural biomedical engineering.
Over the past decade, Minglin Ma, a professor of biological and environmental engineering at Cornell, has tried to develop a better way to control the disease.
In 2017, he unveiled a removable polymer thread containing thousands of islet cells, protected by a thin layer of hydrogel, that could be implanted into a patient’s abdomen. The enclosed islets could secrete insulin in response to falling blood sugar levels while receiving a constant flow of nutrients and oxygen to stay healthy. Ma’s lab created a more robust version in 2021 that was effective in controlling blood sugar levels in diabetic mice for up to six months.
These projects prompted James Shapiro of the University of Alberta in Edmonton to consider a possible collaboration. Shapiro had created a method to insert islets into channels just under a person’s skin, then apply immunosuppression to protect them.
“I was intrigued by the virtues of Ma’s approach because it avoided the need for immunosuppression, and I wondered if we could combine our two innovative strategies to improve cell survival,” Shapiro said. “Indeed, it worked.”
The resulting new system is named SHEATH (Subcutaneous Host-Enabled Alginate THread).
Installation is a two-step process. First, a series of nylon catheters are inserted under the skin, where they remain for four to six weeks, long enough for blood vessels to form around the catheters. When the catheters are removed, the islet devices, which are approximately 10 centimeters long, are inserted into the pocket of space created by the catheters, and the surrounding vasculature remains intact.
“This chain fits our device perfectly,” Ma said. “Putting something under the skin is much easier and much less invasive than in the abdomen. It can be done on an outpatient basis, so you don’t need to stay in the hospital. This can be done under local anesthesia.”
Although additional challenges remain for the long-term clinical application of the device, Ma hopes that future versions can last two to five years before needing replacement.
“The challenge is that it is very difficult to keep these islets functional for a long time inside the body where you have a device, because the device blocks the blood vessels, but we know that the native islet cells of the “Our bodies are in direct contact with vessels that deliver nutrients and oxygen,” said Ma. “The device is designed to maximize the mass exchange of nutrients and oxygen, but we may need to -be providing additional means to support cells for long-term function.”
More information:
Long-Hai Wang et al, Inflammation-induced subcutaneous neovascularization for long-term survival of encapsulated islets without immunosuppression, Natural biomedical engineering (2023). DOI: 10.1038/s41551-023-01145-8
Provided by Cornell University
Quote: An implant under the skin could treat type I diabetes (2023, December 5) retrieved December 5, 2023 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.