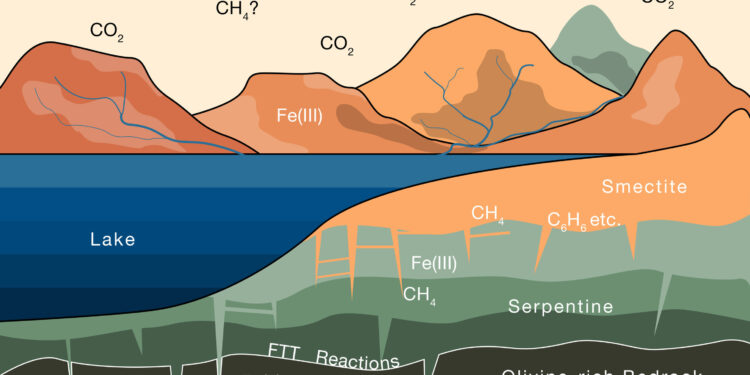
This diagram illustrates the progressive weathering of iron-rich rocks on Mars as the rocks interact with CO2-containing water.2 from the atmosphere. Over several billion years, this process could have stored enough CO2 on the surface of the clay, in the form of methane, to explain most of the CO2 that disappeared from the planet’s primitive atmosphere. Credit: Joshua Murray, Oliver Jagoutz, et al
Mars wasn’t always the cold desert we know today. There’s growing evidence that water flowed across the surface of the Red Planet billions of years ago. And if there was water, there must have been a thick atmosphere to keep that water from freezing. But about 3.5 billion years ago, the water dried up and the air, once laden with carbon dioxide, thinned considerably, leaving behind only the thin wisp of atmosphere that clings to the planet today.
Where exactly did Mars’ atmosphere go? This question is one of the central mysteries of the Red Planet’s 4.6 billion-year history.
For two MIT geologists, the answer may lie in the planet’s clay. In a paper published in Scientific progressThey suggest that much of Mars’ missing atmosphere may be locked away in the planet’s clay crust. The study’s lead author is Joshua Murray, Ph.D. ’24, a recent EAPS graduate.
The team argues that even if water were present on Mars, the liquid could have seeped through certain types of rock and set off a slow chain of reactions that would have gradually pulled carbon dioxide from the atmosphere and turned it into methane, a form of carbon that could be stored for eons in the planet’s clay surface.
Similar processes occur in some regions of Earth. The researchers took their knowledge of the interactions between rocks and gases on Earth and applied it to how similar processes might occur on Mars. They found that, given the amount of clay that covers the surface of Mars, the planet’s clay could hold up to 1.7 bars of carbon dioxide, which would be equivalent to about 80% of the planet’s initial atmosphere.
It is possible that this sequestered Martian carbon could one day be recovered and converted into propellant to power future missions between Mars and Earth, the researchers propose.
“Based on our findings on Earth, we show that similar processes likely took place on Mars and that large amounts of atmospheric CO2 “The methane could have transformed into methane and been sequestered in the clays,” says study author Oliver Jagoutz, a professor of geology in MIT’s Department of Earth, Atmospheric, and Planetary Sciences (EAPS). “This methane could still be present and perhaps even be used as an energy source on Mars in the future.”
In the folds
Jagoutz’s group at MIT seeks to identify the geological processes and interactions that determine the evolution of Earth’s lithosphere, the hard, brittle outer layer that includes the crust and the upper mantle, where tectonic plates reside.
In 2023, he and Murray focused on a type of surface clay mineral called smectite, which is known to be a very effective trap for carbon. Within a single grain of smectite are a multitude of folds, within which carbon can remain intact for billions of years. They showed that smectite on Earth was likely the product of tectonic activity, and that once exposed on the surface, the clay minerals acted to attract and store enough carbon dioxide from the atmosphere to cool the planet for millions of years.
Shortly after the team presented its results, Jagoutz happened to look at a map of the surface of Mars and realized that much of the planet’s surface was covered in the same smectite clays. Could these clays have had a similar carbon-trapping effect on Mars, and if so, how much carbon might these clays contain?
“We know this process is happening and it’s well documented on Earth. And these rocks and clays exist on Mars,” Jagoutz says. “So we wanted to try to connect the dots.”
“Every nook and cranny”
Unlike on Earth, where smectite is a consequence of the shifting and uplifting of continental plates that brought mantle rocks to the surface, there is no such tectonic activity on Mars. The team looked for ways in which the clays could have formed on Mars, based on what scientists know about the planet’s history and composition.
For example, some remote measurements of the surface of Mars suggest that at least part of the Earth’s crust contains ultramafic igneous rocks, similar to those that produce smectites through erosion on Earth. Other observations reveal geological patterns similar to those of terrestrial rivers and tributaries, where water may have flowed and reacted with the underlying rock.
Jagoutz and Murray wondered whether water could have reacted with Mars’ deep ultramafic rocks in a way that would have produced the clays that cover the surface today. They developed a simple model of the rock’s chemistry, based on what is known about how igneous rocks interact with their environment on Earth.
They applied this model to Mars, where scientists believe the Earth’s crust is mostly olivine-rich igneous rocks. The team used this model to estimate how the olivine-rich rocks might change, assuming that water had existed on the surface for at least a billion years and that the atmosphere was loaded with carbon dioxide.
“At this point in Mars’ history, we think that CO2 is everywhere, in every nook and cranny, and the water that seeps through the rocks is full of CO2 “Also,” Murray said.
Over about a billion years, water seeping into the Earth’s crust would have slowly reacted with olivine, a mineral rich in a reduced form of iron. Oxygen molecules in the water would have bonded with the iron, releasing hydrogen and forming the red oxidized iron that gives the planet its iconic color.
This free hydrogen would then have combined with carbon dioxide in the water to form methane. Over time, this reaction allowed the olivine to slowly transform into another type of iron-rich rock, serpentine, which then continued to react with the water to form smectite.
“These smectite clays have a great capacity to store carbon,” Murray says. “So we took existing knowledge about how these minerals are stored in clays on Earth and extrapolated to say: if the Martian surface has this much clay, how much methane can be stored in those clays?”
He and Jagoutz discovered that if Mars is covered with a 1,100-meter-thick layer of smectite, that amount of clay could store a huge amount of methane, equivalent to most of the carbon dioxide in the atmosphere that would have disappeared since the planet dried out.
“We find that estimates of global clay volumes on Mars are consistent with a significant fraction of Mars’ initial CO.2“Mars’ missing atmosphere could be hidden in plain sight,” Murray says.
More information:
Joshua Murray, Olivine weathering and loss of early atmospheric carbon from Mars, Scientific progress (2024). DOI: 10.1126/sciadv.adm8443. www.science.org/doi/10.1126/sciadv.adm8443
Provided by the Massachusetts Institute of Technology
This article is republished with kind permission from MIT News (web.mit.edu/newsoffice/), a popular site covering the latest research, innovation, and teaching at MIT.
Quote:Study shows thick atmosphere of early Mars may be trapped in planet’s clay surface (2024, September 25) retrieved September 25, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.