pH is associated with covariation of denitrification pathway composition in the global topsoil microbiome. Credit: Microbiology of nature (2024). DOI: 10.1038/s41564-024-01752-4
Although a founding concept of ecology suggests that the physical environment determines where organisms can survive, modern scientists suspect there is more to the story behind how microbial communities form in soil.
In a new study, researchers determined, through both statistical analysis and experiments, that soil pH is a key determinant of microbial community composition, but that the need to address toxicity released during the nitrogen cycle ultimately shapes the final microbial community.
“The physical environment affects the nature of microbial interactions, and that affects community assembly,” said Karna Gowda, co-senior author and assistant professor of microbiology at Ohio State University. “People in the field understood that these two things must be important at some level, but there wasn’t a lot of evidence to prove it. We’re adding some specificity and mechanisms to that idea.”
This work helps clarify the microbial underpinnings of the global nitrogen cycle and could provide a new way of thinking about emissions of nitric oxide, a potent greenhouse gas, Gowda said.
The research was recently published in Microbiology of nature.
Microbes keep soil healthy and productive by recycling nutrients and play a particularly important role in converting nitrogen into forms that can be used by plants. Below-ground organisms living in the same environment are also closely interconnected, feeding on each other, participating in chemical exchanges, and providing benefits to the community.
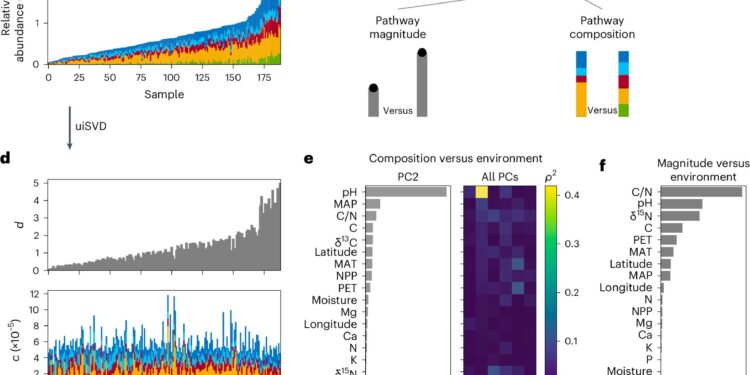
For this work, Gowda and his colleagues used a dataset from a global collection of topsoil samples, sequencing the genomes of microbes present in the samples and analyzing important soil characteristics, such as nitrogen and carbon content and pH, a measure of soil acidity.
“We wanted to look at trends that were widespread and occurring across the planet in very different environments,” Gowda said.
With billions of bacteria present in a soil sample, researchers relied on the genetic makeup of microbial communities to determine their functional roles.
The team focused on genes that helped identify bacteria involved in denitrification, which is the conversion of nitrogen compounds from bioavailable forms to nitric oxide and nitrogen gases released into the atmosphere. A bioinformatics analysis showed that soil pH was the most important environmental factor associated with the abundance of these organisms.
To test these statistical results, the researchers conducted enrichment experiments in the laboratory, passing a natural microbial community through different growth conditions.
In denitrification, specific enzymes play a role in converting nitrate into various nitrogen compounds. One of these forms, nitrite, is more toxic in acidic soil (low pH) than in neutral conditions with higher pH.
Experiments showed that strains containing enzymes called Nar, linked to the creation of toxic nitrites, and strains containing enzymes called Nap, linked to the consumption of nitrites, fluctuated depending on the acidity of the soil.
“We found more Nar at low pH and less Nap, and vice versa as the soil pH approached neutral,” Gowda said. “So we’re seeing two different types of organisms prevailing at acidic versus neutral pH, but we’re also seeing that this doesn’t really explain what’s happening. It’s not just the environment that determines who’s present, it’s actually the environment and the interactions between other organisms in the community.”
“This means that pH affects the interaction between organisms in the community in a more or less constant way: it’s always nitrite toxicity. And it highlights how different bacteria work together to thrive in varying soil pH levels.”
The finding is new and important, Gowda said. Bacteria and other microorganisms are known to be driven by a desire to survive, but they also depend on each other to stay safe. That cooperation has implications for environmental health, the study suggests.
“Although individual fitness effects clearly play a role in defining patterns in many contexts, interactions are likely critical to explaining patterns in a variety of other contexts,” the authors wrote.
Understanding how interactions and the environment affect nitrous oxide emissions could provide new insights into reducing this potent greenhouse gas, Gowda said. Denitrifying bacteria are key sources and sinks of nitrous oxide in agricultural soils. While past studies have focused on how these nitrous oxide-emitting organisms behave under different pH conditions, examining their ecological interactions could offer new strategies for reducing emissions.
More information:
Kyle Crocker et al., Environment-dependent interactions shape patterns of gene content in natural microbiomes, Microbiology of nature (2024). DOI: 10.1038/s41564-024-01752-4
Provided by Ohio State University
Quote:Soil pH determines microbial community composition: Study shows how bacteria work together to thrive in harsh conditions (2024, September 13) retrieved September 13, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.