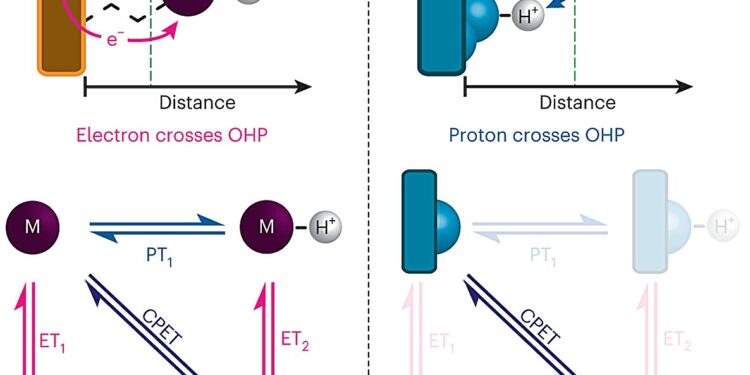
Differences between proton-coupled electron transfer in the outer sphere and at the interface. Top: Illustrations representing the two PCET paradigms: outer sphere (left) and interfacial (right). In OS-PCET, electrons pass through the OHP while in I-PCET, protons pass through the OHP. Bottom: general square diagrams for PCET. OS-PCET can proceed in stages or through concerted pathways, while I-PCET proceeds only through CPET. Credit: Natural chemistry (2024). DOI: 10.1038/s41557-023-01400-0
A key chemical reaction, in which the movement of protons between the surface of an electrode and an electrolyte results in an electric current, is a crucial step in many energy technologies, including fuel cells and electrolyzers used to produce electricity. hydrogen gas.
For the first time, MIT chemists have mapped in detail how these proton-coupled electron transfers occur on the surface of an electrode. Their results could help researchers design more efficient fuel cells, batteries or other energy technologies.
The research paper is published in the journal Natural chemistry.
“Our advance in this paper has been to study and understand the nature of how these electrons and protons couple at a surface site, which is relevant to important catalytic reactions in the context of energy conversion devices or catalytic reactions,” explains researcher Yogesh Surendranath. professor of chemistry and chemical engineering at MIT and lead author of the study.
Among their findings, the researchers were able to trace exactly how changes in the pH of the electrolyte solution surrounding an electrode affect the speed of proton movement and the flow of electrons in the electrode.
MIT graduate student Noah Lewis is the lead author of the paper. Ryan Bisbey, former MIT postdoctoral fellow; Karl Westendorff, MIT graduate student; and Alexander Soudackov, a researcher at Yale University, are also authors of the article.
Passage of protons
Proton-coupled electron transfer occurs when a molecule, often water or an acid, transfers a proton to another molecule or to the surface of an electrode, which stimulates the proton acceptor to also absorb an electron. This type of reaction has been exploited for numerous energy applications.
“These proton-coupled electron transfer reactions are ubiquitous. They are often key steps in catalytic mechanisms and are particularly important for energy conversion processes such as hydrogen generation or fuel cell catalysis “, says Surendranath.
In a hydrogen-generating electrolyzer, this approach is used to remove protons from water and add electrons to the protons to form hydrogen gas. In a fuel cell, electricity is produced when protons and electrons are removed from hydrogen gas and added to oxygen to form water.
Proton-coupled electron transfer is common in many other types of chemical reactions; for example, carbon dioxide reduction (the conversion of carbon dioxide into chemical fuels by the addition of electrons and protons). Scientists have learned a lot about how these reactions occur when the proton acceptors are molecules, because they can precisely control the structure of each molecule and observe how electrons and protons pass between them.
However, when the transfer of proton-coupled electrons occurs at the surface of an electrode, the process is much more difficult to study because electrode surfaces are typically very heterogeneous and have many different sites at which a proton could potentially bind.
To overcome this obstacle, the MIT team developed a way to design electrode surfaces that allows them to much more precisely control the composition of the electrode surface. Their electrodes are made of graphene sheets with organic compounds containing rings attached to the surface. At the end of each of these organic molecules is a negatively charged oxygen ion that can accept protons from the surrounding solution, causing an electron to flow from the circuit to the graphitic surface.
“We can create an electrode that does not consist of a large diversity of sites, but is a uniform set of a single type of very well-defined sites that can each bind to a proton with the same affinity,” says Surendranath. “As we have very well-defined sites, this has allowed us to really understand the kinetics of these processes.”
Using this system, the researchers were able to measure the flow of electrical current to the electrodes, which allowed them to calculate the rate of proton transfer to the oxygen ion at the surface at equilibrium – the state in which the rates of proton donation to the surface and the transfer of protons to the solution from the surface are equal. They found that the pH of the surrounding solution has a significant effect on this rate: the highest levels are at the ends of the pH scale: pH 0, the most acidic, and pH 14, the most basic.
To explain these results, the researchers developed a model based on two possible reactions that could occur at the electrode. In the first, hydronium ions (H3Oh+), which are in high concentration in strongly acidic solutions, deliver protons to the surface of oxygen ions, generating water. In the second, water delivers protons to surface oxygen ions, generating hydroxide ions (OH–), which are in high concentration in strongly basic solutions.
However, the rate at pH 0 is about four times faster than at pH 14, in part because hydronium gives up protons at a faster rate than water.
A reaction to reconsider
The researchers also discovered, to their surprise, that the two reactions have equal rates not at neutral pH 7, where the concentrations of hydronium and hydroxide are equal, but at pH 10, where the concentration of ions hydroxide is 1 million times that of hydronium. The model suggests that this is because the forward reaction involving the donation of protons from hydronium or water contributes more to the overall rate than the reverse reaction involving the removal of protons from water or water. hydroxide.
Existing models of how these reactions occur at the electrode surface assume that forward and backward reactions contribute equally to the overall rate. The new results therefore suggest that these models may need to be reconsidered, the researchers say.
“This is the default assumption, that forward and reverse reactions contribute equally to the reaction rate,” says Surendranath. “Our finding is really eye-opening because it means that the hypothesis that people use to analyze everything from fuel cell catalysis to the evolution of hydrogen might be something we need to revisit.”
The researchers are now using their experimental setup to study how adding different types of ions to the electrolyte solution surrounding the electrode can speed up or slow down the flow of electrons coupled to protons.
“With our system, we know that our sites are constant and do not influence each other, so we can read the effect of the change in the solution on the reaction at the surface,” says Lewis.
More information:
Noah B. Lewis et al, A molecular-level mechanistic framework for interfacial proton-coupled electron transfer kinetics, Natural chemistry (2024). DOI: 10.1038/s41557-023-01400-0
Provided by the Massachusetts Institute of Technology
This story is republished courtesy of MIT News (web.mit.edu/newsoffice/), a popular site that covers news about MIT research, innovation and education.
Quote: Study reveals reaction at the heart of many renewable energy technologies (January 16, 2024) retrieved January 16, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.