by Instituto Nacional de Ciência e Tecnologia de Biologia Estrutural e Bioimagem (INBEB)
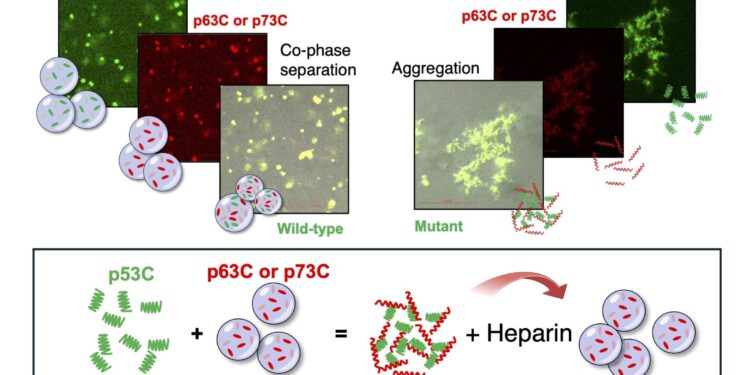
Schematic illustrating the effects of p53C and M237I in the conversion of p63C and p73C droplets into amyloid aggregates at physiologically relevant temperatures. Heparin prevents the formation of p53C/p63C and p53C/p73C aggregates. At 4°C, phase separation occurs without phase transition. Credit: Guilherme Oliveira
Research led by the Federal University of Rio de Janeiro (UFRJ) and the National Institute of Science and Technology of Structural Biology and Bioimaging (INBEB) in Brazil has uncovered a critical mechanism by which mutations in the p53 protein, a key tumor suppressor known as the “guardian of the genome,” turn other proteins into cancer-promoting agents.
The study, led by Dr. Jerson Lima Silva, offers new insights into a process that plays a critical role in the development and progression of many cancers. The findings were recently published in Communication Chemistry.
p53 is essential to the body’s defense against cancer. It is responsible for regulating the cell cycle and triggering the death of damaged cells before they become malignant. However, in more than 50% of tumors, mutations in p53 compromise its protective role, transforming it into a cancer driver. In this study, the research team demonstrated that mutant p53 not only loses its tumor-suppressing abilities, but also gains the ability to corrupt other antitumor proteins, including p63 and p73.
Through a mechanism known as aberrant phase transition, mutant p53 induces amyloid aggregation in p63 and p73, forming clusters of harmful proteins called amyloid structures. These aggregates cause these proteins to become oncogenic, leading to uncontrolled tumor growth. The study also suggests that other proteins may be affected in a similar way, although further research is needed to identify them.
These findings provide crucial new insights into why some tumors, such as glioblastoma, are so aggressive. They also pave the way for the development of targeted cancer treatments that could disrupt or reverse the harmful interactions caused by mutant p53.
Full-length exogenous p53 M237I protein colocalizes with endogenous p73 protein. Credit: Communication Chemistry (2024). DOI: 10.1038/s42004-024-01289-x
“This discovery could be critical for designing therapies for aggressive cancers, including glioblastoma, in which mutant p53 plays a dominant role,” said Dr. Silva.
Using advanced biophysical techniques and fluorescence microscopy, the team studied three specific mutations in the p53 gene: M237I, commonly seen in glioblastoma; R249S, which is prevalent in liver cancer; and R248Q, associated with breast cancer. They found that each of these mutants not only altered the behavior of the p53 gene, but also triggered the formation of amyloid-like structures by the p63 and p73 genes, thereby promoting cancer progression.
“While normal p53 can form functional biomolecular condensates,” Silva explains, “these mutants dramatically accelerate the transition to solid, amyloid-like states. This change occurs through a prion-like mechanism, converting p63 and p73 condensates into aggregates. This process may be crucial for understanding how p53 mutations contribute to cancer development.”
Interestingly, research has also revealed that heparin, a widely used anticoagulant, can inhibit the formation of these harmful aggregates. This suggests a potential therapeutic approach to prevent or reverse the malignancy-promoting effects of the mutant p53 gene.
“This discovery opens a new frontier to target cancer at its roots, by intervening in the phase transitions and aggregation of p53, p63 and p73,” added Dr. Silva.
More information:
Elaine C. Petronilho et al, Oncogenic p53 triggers amyloid aggregation of p63 and p73 liquid droplets, Communication Chemistry (2024). DOI: 10.1038/s42004-024-01289-x
Provided by the Instituto Nacional de Ciência e Tecnologia de Biologia Estrutural e Bioimagem (INBEB)
Quote:Study reveals how mutant p53 protein converts other proteins into cancer drivers (2024, September 16) retrieved September 16, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.