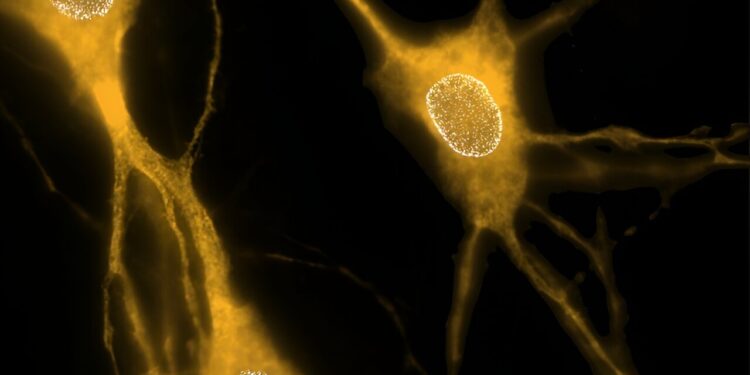
This image shows the distribution of nuclear pore complexes (NPCs) in three isolated neurons. NPCs are groups of proteins that form nuclear envelope pores, which are openings that allow proteins and genetic material to move between the cell nucleus and the cytoplasm. Credit: UT Southwestern Medical Center
A protein called torsinA plays a key role in early neuron development by determining where nuclear pores are placed in the membrane that surrounds the nucleus of nerve cells, a study by researchers at UT Southwestern Medical Center shows.
The results, published in Natural Cell Biologyanswer long-standing questions about the function of torsinA and could lead to treatments for a rare movement disorder called DYT1 dystonia, caused by a mutation in torsinA.
“The function of torsinA has remained unclear. This work demonstrates that torsinA is involved in the spatial organization of nuclear pore complexes and suggests that dysregulation of this process during neurodevelopment could contribute to lasting neuronal deficits,” said Samuel Pappas, Ph.D., assistant professor in the Peter O’Donnell Jr. Brain Institute and of Neurology at UT Southwestern.
Dr. Pappas co-led the study with Dr. William Dauer, director of the O’Donnell Brain Institute and professor of neurology and neuroscience, and Dr. Sami Barmada, Ph.D., associate professor of neurology at the University of Michigan. The study’s first author is Sumin Kim, Ph.D., a former graduate student at the University of Michigan who was co-supervised by Drs. Pappas, Dauer and Barmada.
DYT1 dystonia is characterized by abnormal twisting and shaking of the arms and legs that begins in childhood. Epidemiological studies have estimated that this inherited condition affects between 54,000 and 80,000 people in the United States. However, nearly three times that number carry the torsin A genetic mutation that causes DYT1 dystonia.
Questions about why symptoms appear during this time, which torsin A mutations cause them, and why so many carriers are not affected remain unanswered. Torsin A function is a key piece in solving these mysteries, Pappas said.
In previous studies, he and his colleagues learned that deleting the gene that produces torsinA early in development in animal models, but not in adulthood, caused neurons to develop nuclear pore complexes (NPCs)—collections of proteins that form the pores in the nuclear envelope—that were clustered together, rather than distributed across the nuclear membrane.
These tiny openings in the nuclear envelope allow proteins and genetic material to move between the cell nucleus and the fluid that fills cells, called the cytoplasm.
To determine how torsinA might be involved in NPC distribution, Drs. Pappas, Dauer, Barmada, and their colleagues tracked the number and placement of these structures in neurons developing in Petri dishes isolated from animals shortly after birth.
Within the first few days of isolation, these cells produced many more neuronal cells, consistent with the maturation of neuronal circuits. Additional experiments on cultured neurons demonstrated that deleting torsin A from these cells did not change the number of neuronal cells, but it did change their position within the nuclear envelope, which caused the clustering the researchers observed in the previous study.
To study the function of torsin A in living animals, the researchers developed a special mouse strain in which a CNP protein was tagged with a fluorescent marker. They found that the clumps consisted of newly formed CNPs that were not evenly distributed across the nuclear membrane.
Further research showed that neurons in which the torsinA gene had been deleted or replaced with the same mutated form carried by DYT1 dystonia patients developed abnormal swollen areas in the nuclear envelope before the appearance of NPCs during maturation.
These “bubbles” were located in the same locations where the neural stem cell clusters were found. Using a technique called super-resolution microscopy, the researchers showed that the resulting neural stem cells appeared to have a normal conformation, albeit with an abnormal, clustered distribution within the nuclear envelope.
Together, Dr. Pappas explained, these results suggest that torsinA controls the final location of CNPs during a critical period early in neuron development—a step that appears essential for proper neuron function later in life and that corresponds to the typical timing of the onset of DYT1 dystonia symptoms.
Further study could help scientists understand what differentiates people who carry the disease-causing mutation and develop symptoms from those who never develop them. Eventually, he said, these findings could lead to therapies to treat the disease.
“Our previous work has shown that alteration of torsin A, such as that which occurs in dystonia, plays a critical role in the formation of the central nervous system during a critical period of development,” said Dr. Dauer.
“This new work identifies nuclear pore biogenesis as a torsinA-dependent process during this critical period, so it is exciting to establish a mechanistic approach to overcome this defect.”
More information:
Sumin Kim et al, TorsinA is essential for neuronal nuclear pore complex localization and maturation, Natural Cell Biology (2024). DOI: 10.1038/s41556-024-01480-1
Provided by UT Southwestern Medical Center
Quote:Study reveals how a key protein affects the structure of neurons (2024, September 12) retrieved September 12, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.