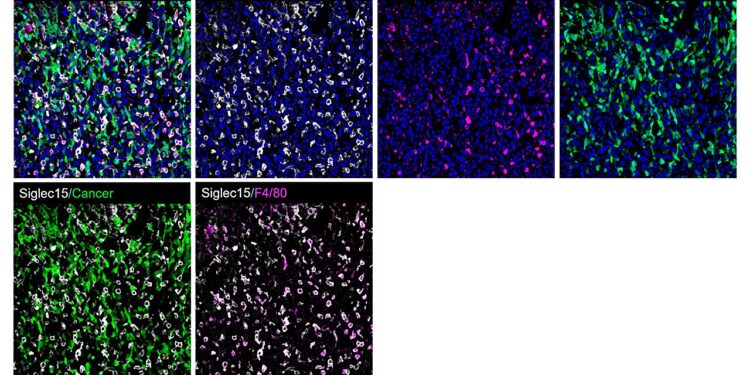
Representative immunofluorescence staining of the metastatic bone niche of mouse-bearing LLC1 bone metastases (tumor region). Macrophage marker F4/80 (red), cancer (green), Siglec-15 (white) and nuclei (blue). Credit: Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2312929121
Researchers in the laboratory of chemist Han Xiao at Rice University have identified a promising new immunological pathway for treating stubborn bone tumors, one of the most common forms of metastasis in breast cancer patients.
“More than 70% of people with metastatic breast cancer will have cancer cells move to the bones, which can lead to skeletal-related events like bone pain, fractures and hypercalcemia,” said Yixian Wang , a Rice graduate student in the Han lab. who is the lead author of a study published in Proceedings of the National Academy of Sciences.
“There are now several immunotherapies that can potentially benefit breast cancer patients with metastases, but they are not effective in patients with bone tumors.”
Each year, more than 240,000 new cases of breast cancer are diagnosed in the United States, according to the Centers for Disease Control and Prevention. About a quarter of these patients will experience metastasis, where cancer cells spread from the breast to other parts of the body.
New immunotherapies called checkpoint inhibitors can uncover stubborn tumors, allowing the immune system to send powerful immune cells to treat the abnormal cells. But even though checkpoint inhibitors are effective for many patients, they don’t work for everyone, and clinical trials have shown little or no response when used to treat bone metastases.
Yixian Wang (left) and Han Xiao. Credit: Gustavo Raskosky/Rice University
Xiao, an associate professor of chemistry, biosciences and bioengineering at Rice, said he and his team wanted to find another route that might be more effective at erasing these stubborn bone metastases.
“We thought there must be another new checkpoint axis that we could target for breast cancer cells in bone,” Xiao said. “And we discovered a unique glycoimmune checkpoint axis in bone metastases that involves a sialic acid-binding protein called Ig-like lectin (Siglec)-15. We learned that it suppresses immune cells in the bone.”
After noting a significant increase in Siglec-15 in the tumor microenvironment in bone tumor samples from breast cancer patients, Xiao and colleagues demonstrated that this receptor plays an important role in hiding bone tumors from immune surveillance.
“Checkpoint inhibitors currently approved by the FDA are mediated by protein-protein interactions that suppress immune cells,” Xiao explained. “Siglec-15, however, is a glyco-immune checkpoint inhibitor. Instead of binding to a protein, Siglec-15 binds to sugars that you find on the surface of cells and that’s how “It can suppress the immune system. This is a whole other problem. New type of immune checkpoint that offers great promise for the future treatment of bone cancers.”
Xiao’s team conducted several cell culture experiments to study Siglec-15 interactions in the bone tumor microenvironment. They learned that it is involved in crosstalk between tumor cells and important immune cells like T lymphocytes and macrophages, as well as bone-specific cells called osteoclasts.
“You can find these glycolipids and glycoproteins on all cells and we know they play an important role in immune modulation,” Xiao added. “These results provide us with the opportunity to further study these glycoimmune checkpoint inhibitors and identify those that may help bone tumors stop evading immune recognition.”
But simply modulating the behavior of Siglec-15 could be enough to treat bone metastases. When the team injected a monoclonal antibody targeting Siglec-15 into an animal model of metastatic breast cancer with bone tumors, they were able to trigger a powerful immune response. In fact, researchers saw tumors shrink after just one or two doses of antibody treatment.
“It was really a striking finding,” Wang said. “I am very excited about the potential therapeutic outcomes of a therapy like this. It could be a very useful treatment for breast cancer patients in the future.”
Xiao said he and his team plan to continue studying the novel and unique biology of glycoimmune control pathways in the tumor microenvironment. He added that there is still much to learn about these pathways and that future studies should provide new biological insights that may improve current and future immunotherapies.
Additionally, Xiao said he would like to see if targeting Siglec-15 could be useful in treating other types of cancers affecting bone.
More information:
Yixian Wang et al, Siglec-15/sialic acid axis as a central glycoimmune checkpoint in breast cancer bone metastases, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2312929121
Provided by Rice University
Quote: Study reveals new immunological pathway for treatment of bone metastases from breast cancer (January 24, 2024) retrieved January 24, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.