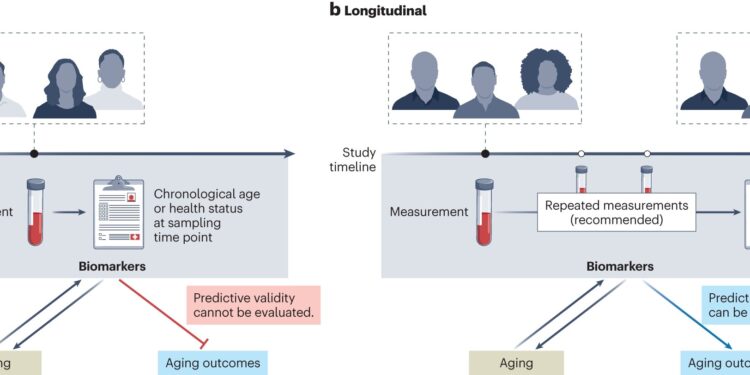
Different approaches to cohort study design in the context of biomarkers of aging. Biomarkers of aging are generally validated using cross-sectional or longitudinal study designs. a, Cross-sectional studies involve the measurement of biomarkers and data on chronological age or aging-related outcomes at a single point in time. These data can only support the combination of these measures at this time. b, Longitudinal designs, on the other hand, allow assessment of the predictive validity of biomarkers measured at a single time point and future aging-related outcomes. Credit: Natural medicine (2024). DOI: 10.1038/s41591-023-02784-9
Biomarkers are measurable characteristics that can be used to assess normal biological processes, diseases, or responses to treatment in patients. The use of biomarkers to assess biological aging, or increasing molecular and cellular damage over time, has recently gained popularity due to its potential to predict longevity and quality of life. However, there are currently no guidelines to standardize the development and validation of biomarkers of aging, a necessary process to ensure accurate and reliable results in the clinic.
A new study led by researchers at Brigham and Women’s Hospital has proposed a framework for future validation of biomarkers of aging that could help translate them into clinically actionable tools.
The work is published in the journal Natural medicine.
To do this, the team looked at population-based cohort studies of blood-based biomarkers of aging constructed using omics data. Using this information, they identified challenges in comparing the predictive strength of biomarkers, such as variations in study design and data collection methods, as well as inherent differences in population-specific traits. .
The authors then made recommendations to resolve these difficulties. They suggest that “multiomics approaches” involving different technologies such as metabolomics, proteomics, epigenetics and transcriptomics will provide better insights into the predictive performance of biomarkers. Instead of relying solely on mortality as an aging-related outcome, researchers advocate considering associations of biomarkers with other health factors such as functional decline, frailty, chronic disease, and disability. Additionally, they recommend normalizing omics data to improve validation efforts.
“Omics and biomarker harmonization efforts, such as the Bio-learn project, are instrumental in validating biomarkers of aging,” said co-first author Mahdi Moqri, Ph.D., of the Division of Genetics.
The framework also encourages increased collaboration between research groups on large-scale longitudinal studies to track long-term physiological changes and responses to treatments in diverse populations. Further work is needed to understand how implementing biomarker assessment in clinical trials could improve patient quality of life and survival.
“If we hope to have clinical trials for interventions that extend healthy lifespan in humans, we need reliable and validated biomarkers of aging,” said co-first author Jesse Poganik, Ph.D. , of the Division of Genetics. “We hope our framework will help prioritize the most promising biomarkers and provide healthcare providers with clinically valuable and actionable tools.”
More information:
Mahdi Moqri et al, Validation of biomarkers of aging, Natural medicine (2024). DOI: 10.1038/s41591-023-02784-9
Provided by Brigham and Women’s Hospital
Quote: Study proposes framework to standardize aging biomarkers and accelerate clinical use (February 14, 2024) retrieved February 14, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.