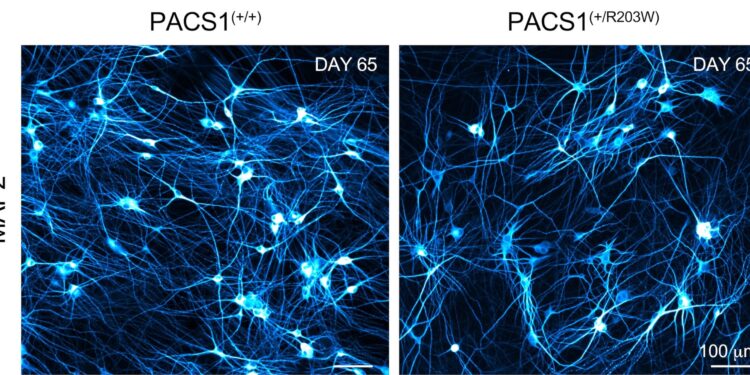
Neurons cultured for 65 days from healthy cells and those containing the PACS1 syndrome variant both have complex morphology and express neuronal microtubule-associated protein 2 (blue). Credit: Lauren Rylaarsdam, Ph.D.
Researchers in the laboratory of Alicia Guemez-Gamboa, Ph.D., assistant professor of neuroscience, have discovered new molecular mechanisms of PACS1 syndrome, a rare neurodevelopmental disorder, according to results published in Natural communications.
PACS1 syndrome is characterized by intellectual disability and distinct craniofacial abnormalities. Patients may also have epilepsy, autism, hypotonia (muscle weakness), feeding difficulties, and heart defects. The disease, first identified in 2012, is caused by a single sporadic variant of the PACS1 (phosphofurin acid cluster sorting 1) gene.
“Most neurodevelopmental disorders result from many different variants, but with PACS1 syndrome, all patients have the same one, so it’s a pretty unique situation,” said former graduate student Lauren Rylaarsdam, Ph.D. from the Guemez-Gamboa laboratory and lead author of the study.
There are approximately 200 known cases of PACS1 syndrome, most of which have been reported in children, although recent advances in genetic sequencing technology may help identify more patients.
“Unfortunately, there is no treatment available, so we teamed up with the PACS1 Syndrome Research Foundation to try to understand how the variant has impacted brain development and how to fix it,” Guemez-Gamboa said.
To do this, the authors obtained reprogrammed stem cells from patients’ skin fibroblasts (cells that help form connective tissue) and also used gene editing tools to introduce the variant causing PACS1 syndrome into cells. healthy and correct it in patient cell lines. The authors then differentiated these cells into neurons to understand how the PACS1 variant impacted the developing brain.
Because little was known about PACS1 syndrome, scientists used a variety of tools, including single-cell RNA sequencing of brain organoids, a cutting-edge 3D culture technique in which many types of neural cells grow simultaneously in small spheres.
Interestingly, they found that mature excitatory neurons appeared more affected than young excitatory neurons. Mature neurons from PACS1 syndrome organoids also showed altered expression of genes related to synaptic signaling, the primary method of communication between neurons in the brain.
“This, in conjunction with the epilepsy and autism that the patients suffer from, led us to think that there might be differences in the firing patterns of PACS1 syndrome neurons,” Rylaarsdam said.
The investigators then captured the activity of PACS1 excitatory neurons using multielectrode arrays, which allowed them to record the activity of multiple cells at once. Using this technique, scientists found that bursts of activity lasted much longer in neurons containing the PACS1 variant.
“This is a big step toward understanding what might contribute to the epilepsy and autism that patients suffer from,” Rylaarsdam said.
In the future, the investigators said they plan to determine whether neurons are similarly affected in mouse models of PACS1 syndrome. Their ultimate goal, according to Rylaarsdam, is to identify and test therapies that repair the phenotype observed in neurons carrying the PACS1 mutation.
“The brain is made up of many diverse regions connected by a complex circuit system. It is therefore important to understand how the increase in burst duration that I observed in a very isolated context correlates with the brain in its together.
“We found preliminary evidence suggesting that inhibitory neurons in the brain are also affected, but our models were better suited to studying excitatory neurons. So this is also an area where the mouse model will be very important,” he said. said Rylaarsdam.
More information:
Lauren Rylaarsdam et al, iPSC-derived models of PACS1 syndrome reveal transcriptional and functional deficits in neuronal activity, Natural communications (2024). DOI: 10.1038/s41467-024-44989-7
Provided by Northwestern University
Quote: Study identifies molecular mechanisms of a rare neurodevelopmental disorder (February 6, 2024) retrieved February 6, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.