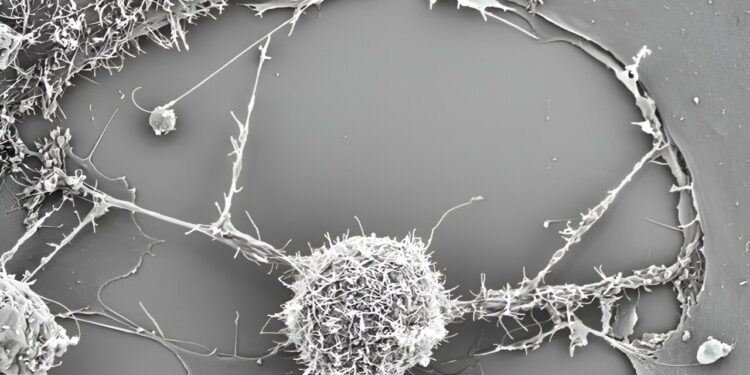
Nanotubes deliver mitochondria to T cells, making them more effective at fighting cancer. Credit: Shiladitya Sengupta, Brigham and Women’s Hospital
Fighting cancer is exhausting for T cells. Hostile tumor microenvironments can deplete their mitochondrial activity, leading to a state known as T cell exhaustion. This phenomenon also hampers adoptive cell therapies, in which healthy tumor-targeting T cells are injected into cancer patients. A new method to boost mitochondrial activity and recharge T cells is needed.
Researchers at Brigham and Women’s Hospital, working with colleagues at the Leibniz Institute for Immunotherapy in Germany, have developed a way to “supercharge” T cells by providing them with extra mitochondria from multipotent stromal cells.
In a study published in CellThe researchers report that these supercharged T cells showed increased antitumor activity and reduced signs of exhaustion in preclinical cancer models, suggesting that this technique could help improve existing immunotherapies.
“These supercharged T cells overcome one of the fundamental barriers to immunotherapy by penetrating the tumor and overcoming the immune-sterile state within the tumor,” said corresponding author Shiladitya Sengupta, Ph.D., of the Brigham Department of Medicine.
“Mitochondria provide the fuel. It’s like taking T cells to the gas station and filling them up with gasoline. This mitochondrial transplantation marks the dawn of organellar therapy where an organelle is delivered to a cell to make it more efficient.”
“Previous efforts to improve T cell mitochondrial function have focused on targeting specific genes or pathways, but these methods are not sufficient when mitochondria are already damaged or dysfunctional. Our approach involves the transfer of whole, healthy mitochondrial organelles into cells. This process is comparable to organ transplantation, such as heart, liver or kidney transplants, but performed at a microscopic level,” explained Dr. Luca Gattinoni, co-senior author of the study.
To develop this method, the researchers built on their previous findings, which showed that cancer cells can suck up mitochondria from immune cells using intercellular nanotubes that the researchers described as “tiny tentacles.”
Building on these findings, the group teamed up with scientists from the Leibniz Institute to study the interactions between bone marrow stromal cells (BMSCs) and cytotoxic T cells. Using various electron microscopy and fluorescence approaches, they observed that BMSCs extended nanotubes to activated T cells, resulting in intact mitochondria. This helped revive the T cells (mito+), which showed increased respiratory capacity, a sign of improved metabolism.
The research team looked at how supercharging T cells affected immune function. When infused into a mouse model of melanoma, the mito+ cells showed significantly higher antitumor responses and prolonged survival rates compared to T cells without additional mitochondria.
Further experiments revealed that mito+ The cells could easily enter the tumors, multiply rapidly, and pass their extra mitochondria to daughter cells, where they persisted for a long time. In addition, the authors found that the mitochondria+ cells could survive and resist T cell exhaustion in the tumor microenvironment.
Researchers have found that supercharging human T cells helps the immune system fight tumors in several cancer models. In particular, they showed that tumor-infiltrating cells and CAR-T cells, which often develop damaged mitochondria in the tumor microenvironment, exhibited enhanced cancer-killing properties when stimulated by mitochondria from human donor embryonic stem cells (BMSCs).
The authors suggest that future applications could include using patient-matched mesenchymal stem cells (BMSCs) to supercharge T cells for adoptive transfer.
More information:
Intercellular nanotube-mediated mitochondrial transfer improves T cell metabolic fitness and antitumor efficacy, Cell (2024). DOI: 10.1016/j.cell.2024.08.029. www.cell.com/cell/fulltext/S0092-8674(24)00956-5
Cell
Provided by Brigham and Women’s Hospital
Quote:Study finds ‘superfeeding’ T cells with mitochondria enhances their antitumor activity (2024, September 13) retrieved September 13, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.