Credit: Current Biology (2024). DOI: 10.1016/j.cub.2024.08.004
Placebos are inert treatments, not typically expected to impact biological pathways or improve a person’s physical health. Yet time and again, patients report feeling better after taking a placebo. Increasingly, doctors and scientists are recognizing that rather than viewing placebos as mere gimmicks, they can help patients by harnessing their power.
To maximize the impact of the placebo effect and design reliable therapeutic strategies, researchers need to better understand how it works. Now, thanks to a new animal model developed by scientists at MIT’s McGovern Institute, they will be able to study the neural circuits that underlie the ability of placebos to cause pain relief.
“The brain-body interaction has tremendous potential, but we don’t fully understand it yet,” says Fan Wang, a professor of brain and cognitive sciences at MIT and a researcher at the McGovern Institute. “I really think we need to do more to understand the placebo effect, in pain and probably in many other conditions. We now have a solid model to study the circuit mechanism.”
Context-dependent placebo effect
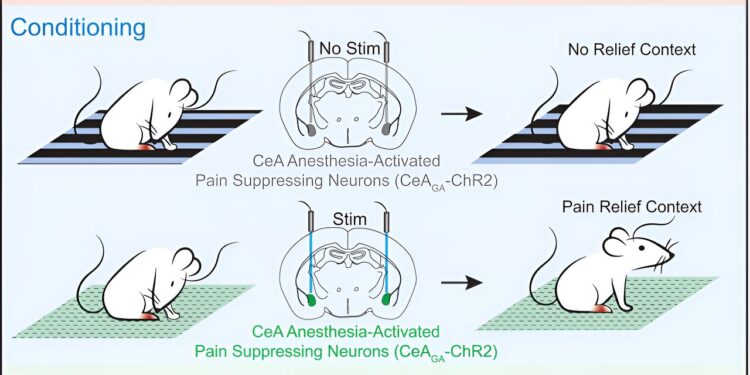
In the September 5, 2024 issue of the journal Current BiologyWang and his team report that they induced strong placebo pain relief in mice by activating pain-suppressing neurons in the brain while the mice were in a specific environment, thereby teaching the animals that they feel better when they are in that context.
After training, placing the mice in this environment alone was enough to eliminate the pain. The team’s experiments show that this context-dependent placebo effect relieves both acute and chronic pain.
Context is key to the placebo effect. If a pill can make a patient feel better when they expect it, even if it is made up of just sugar or starch, it seems that it is not just the pill that elicits these expectations, but the entire scenario in which the pill is taken. For example, being in a hospital and interacting with doctors can contribute to a patient’s perception of care, and these social and environmental factors can make a placebo effect more likely.
MIT postdocs Bin Chen and Nitsan Goldstein used visual and textural cues to define a specific location. They then activated pain neurons in the brain while the animals were in the “pain box.”
These pain neurons, discovered by Wang’s lab a few years ago, are located in an emotion-processing center of the brain called the central amygdala. By expressing light-sensitive channels in these neurons, the researchers were able to suppress pain with light in the pain box and leave the neurons inactive when the mice were in a control box.
The animals learned to prefer the pain box to other environments. And when the researchers tested their response to potentially painful stimuli after making this association, they found that the mice were less sensitive when they were in the box.
“Just by being in the context that they had associated with pain suppression, we saw a reduction in pain, even though we weren’t actually activating those (pain suppressor) neurons,” Goldstein says.
Relief of acute and chronic pain
Some scientists have successfully induced placebo pain relief in rodents by treating the animals with morphine, linking environmental cues to the drugs’ pain suppression, much in the same way that Wang’s team did by directly activating pain-suppressing neurons.
This drug-based approach is particularly effective at creating expectations of relief for acute pain; its placebo effect is short-lived and generally ineffective against chronic pain. Wang, Chen, and Goldstein were therefore particularly pleased to find that their artificial placebo effect was effective in relieving both acute and chronic pain.
In their experiments, animals with chemotherapy-induced hypersensitivity to touch showed a preference for the pain box as much as animals exposed to a chemical that caused acute pain, a few days after their initial conditioning. Once there, their sensitivity to chemotherapy-induced pain was eliminated; they showed no more sensitivity to painful stimuli than they had before receiving chemotherapy.
One of the biggest surprises came when the researchers turned their attention back to the pain neurons in the central amygdala that they had used to trigger pain relief. They suspected that these neurons might be reactivated when the mice returned to the pain box.
Instead, they found that after the initial conditioning period, these neurons remained silent. “These neurons are not reactivated, but the mice no longer appear to be in pain,” Wang says. “So this suggests that this memory of well-being is being transferred elsewhere.”
Goldstein adds that there must be a pain-suppressing neural circuit somewhere that is activated by contexts associated with pain relief — and the team’s new placebo model prepares researchers to study these pathways.
A deeper understanding of this circuit could allow clinicians to deploy the placebo effect, alone or in combination with active treatments, to better manage patients’ pain in the future.
More information:
Bin Chen et al., Reverse engineering of placebo analgesia, Current Biology (2024). DOI: 10.1016/j.cub.2024.08.004
Provided by the Massachusetts Institute of Technology
This article is republished with kind permission from MIT News (web.mit.edu/newsoffice/), a popular site covering the latest research, innovation, and teaching at MIT.
Quote:Harnessing the power of placebo to relieve pain: Study examines neural circuits linked to the effect (September 11, 2024) Retrieved September 11, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.