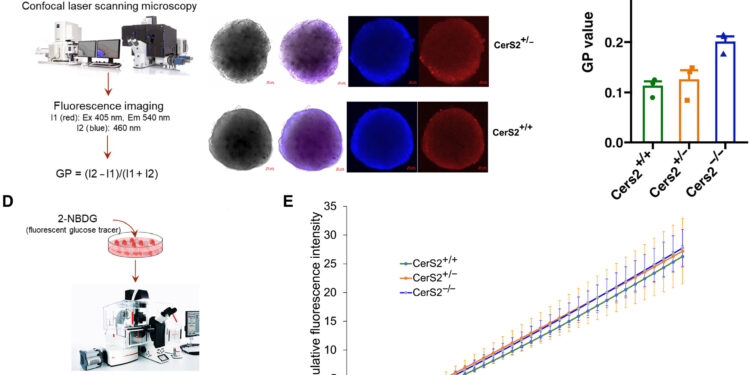
Rigidity of the islet membrane. Credit: Scientific advances (2025). DOI: 10.1126/sciadv.adr1725
Of those who have had gestational diabetes, approximately 35% will develop type 2 diabetes within a decade. New study led by Dr. Saifur Khan, Ph.D., cardiology research faculty member at the University of Pittsburgh Institute for Vascular Medicine, is the first to identify molecular mechanisms of diabetes progression gestational to type 2 diabetes.
The discovery, reported in Scientific advancescould lead to new treatments and interventions aimed at reducing the risk of this progression.
“Many of these women who have had gestational diabetes develop type 2 diabetes at a relatively young age, before age 40,” Khan said. “There is an urgent need to identify root causes in order to develop early detection, prevention and targeted interventions using precision medicine.”
The team examined a cohort from the Study of Women, Infant Feeding, and Type 2 Diabetes After Gestational Diabetes (SWIFT Study), focusing on 143 Hispanic women ages 20 to 45 who had a history of gestational diabetes. Among them, 65 progressed to type 2 diabetes within eight years of giving birth. The remaining 78 women served as controls.
By examining human metabolomics, lipidomics and genomic data, the team found that those who developed type 2 diabetes had reduced levels of a type of lipid called sphingolipids in their blood in their disease-free stage, then followed a decreased production of sphingolipids. to a mutation in a gene known as CERS2. The team validated this discovery in mouse models as well as in experiments using insulin-secreting cells donated by humans.
The results suggest that lower levels of circulating very long-chain sphingolipids may serve as an early indicator of progression from gestational diabetes to type 2 diabetes.
“These findings could pave the way for new therapeutic strategies aimed at targeting the sphingolipid pathway,” said Khan, also of the VA Medical Center in Pittsburgh.
“Therapeutic interventions designed to restore adequate sphingolipid metabolism by enhancing CERS2 activity or minimizing its deleterious downstream effects on pancreatic insulin secretion capacity could help improve pancreatic beta cell function, secretion of Insulin and glucose regulation in individuals at high risk of developing type 2 diabetes, in particular. those with a history of gestational diabetes.
In future studies, the team will explore how loss of function of CERS2 contributes to pancreatic beta cell dysfunction and assess CERS2 activity and sphingolipid metabolism as targets.
Other study authors were Xiangyu Zhang, Ph.D., and Babak Razani, MD, Ph.D., of Pitt and VA Medical Center, Pittsburgh; Wenyue W. Ye, MD, Julie AD Van, Ishnoor Singh, Yasmin Rabiee, Kaitlyn L. Rodricks and Michael B. Wheeler, Ph.D., all of the University of Toronto; Rebekah J. Nicholson and Scott A. Summers, Ph.D., of the University of Utah; Anthony H. Futerman, Ph.D., of the Weizmann Institute of Science; Erica P. Gunderson, MD, Ph.D., of Kaiser Permanente Northern California and the Kaiser Permanente Bernard J. Tyson School of Medicine.
More information:
Saifur R. Khan et al, Reduction in circulating sphingolipids and CERS2 activity are linked to T2DM risk and impaired insulin secretion, Scientific advances (2025). DOI: 10.1126/sciadv.adr1725
Provided by the University of Pittsburgh
Quote: The progression of gestational diabetes towards type 2: a study discovers a genetic predisposition linked to biomarkers (January 14, 2025) retrieved January 14, 2025 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.