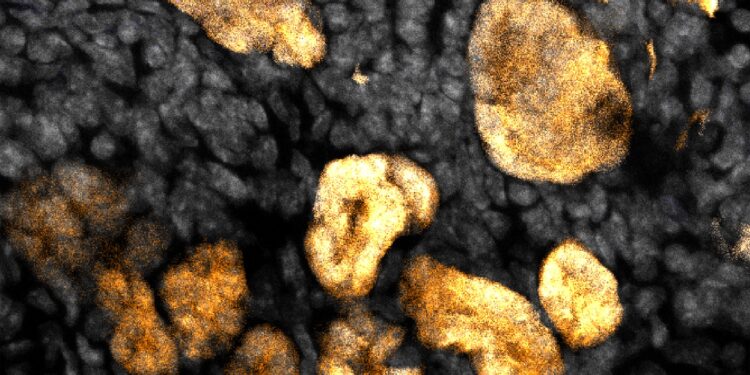
Mouse kidney nephron progenitor cells (purple) forming the tubules of the developing nephron (yellow) of the kidney’s filtering units, the nephrons. Credit: Helena Bugacov/McMahon Lab
A group of essential signaling molecules known as the Wnt pathway emerged early in the evolution of multicellular life. Scientists have studied the actions of Wnt for four decades to understand its complex roles in development and disease.
In the development of the mammalian kidney, USC Stem Cell scientists in Andy McMahon’s laboratory undertook two complementary studies, published in the journal Developmentwhich provide new insights into the critical role of Wnt signaling in initiating mammalian kidney development.
“Many stem and progenitor cells require Wnt signaling, and the kidney is a particularly interesting example, because the level of signaling can have profoundly different outcomes,” said corresponding author McMahon, director of WM Keck and University Professor of Stem Cell Biology and Regenerative Medicine and Biological Sciences at the Keck School of Medicine of USC.
“By improving our knowledge of how Wnt signaling acts in the developing kidney, these two papers provide information that can guide the efforts of USC collaborators in the Synthetic Kidney Consortium to build kidneys from cells stems and progenitors as a new treatment option for patients.
Both studies focus on the progenitor and stem cells that form the kidney’s filtering units, called nephrons, in embryonic mice.
“Nephron progenitor cells cease to exist the moment humans are born,” said Helena Bugacov, first author of both studies and a Ph.D. graduate of the McMahon Lab, she is currently pursuing her MD at the Icahn School of Medicine at Mount Sinai in New York.
“Without NPCs, postnatal kidneys are unable to form new nephrons, hence the need for kidney transplantation when nephron function declines. However, there are simply not enough kidneys available for those who do. need.
“Therefore, understanding the signals necessary to promote self-renewal, differentiation and the formation of nephron precursors from their progenitor cells is essential for the creation of stem cell-based artificial kidneys.”
The scientists isolated and cultured nephron progenitor cells, or NPCs, in the laboratory, then exposed them to different amounts of a chemical called CHIR, which changes the activity of the Wnt signaling pathway.
To explore the actions of the Wnt pathway, researchers focused on how Wnt signals regulate genes, a process mediated by proteins involved in DNA binding. In addition to its essential role in Wnt-mediated gene regulation, beta-catenin is a key mediator of cell adhesion processes that create and hold together a type of tissue called epithelium.
To study the actions of these components of the Wnt pathway, Helena Bugacov developed a technique for genetic manipulation of NPCs.
In the first study, Bugacov and colleagues applied the genetic modification technique to study responses to different levels of Wnt pathway activation.
The scientists discovered that low levels of signaling regulate NPC self-renewal, essential for generating the full number of NPCs needed to form the 14,000 nephrons in the mouse kidney. Higher levels initiate differentiation of NPCs into mature kidney cell types. Consistent with previous studies from the McMahon Lab and others, beta-catenin levels determine different outcomes for NPCs.
Induction of kidney formation in response to high levels of Wnt signaling results in a critical cellular transition: isolated NPCs aggregate and cooperatively form a small group of cells, called a kidney vesicle. Each renal vesicle is the precursor of a single nephron. A million renal vesicles generate the human’s million nephrons.
In the second study, the first authors – postdoc Bálint Dér, MD, and Bugacov of the McMahon Lab – investigated how the Wnt signaling pathway directs the aggregation of NPCs to form the condensed clusters that become precursors to nephrons.
Dér, Bugacov and their co-authors discovered that Wnt activation causes NPCs to adhere to each other, transforming from a mobile, loosely organized collection of cells into a stationary, organized arrangement of cells that forms the vesicle renal.
This process, known as mesenchymal-epithelial transition, is a hallmark of embryonic development in the kidney, as well as many other developmental and disease processes throughout the body. The reverse process, an epithelial-mesenchymal transition, is responsible for the spread of many cancers from the primary tumor to distant sites during tumor metastasis.
To achieve this cellular aggregation that allows nephrons to begin to take shape, beta-catenin connects adhesive proteins on the surface of NPCs, called cadherins, through another protein, alpha-catenin, to a structural scaffolding within the cell.
“It has been a pleasure and honor to work on these research projects in a laboratory that has studied the Wnt signaling pathway since the first identification of Wnt genes and their developmental actions in mammals, and to be able to combine the power of development, stem cell science and genetic engineering to one day advance treatment options for people with kidney disease,” Bugacov said.
Dér, who currently specializes in urological surgery at Semmelweis University in Budapest, Hungary, added: “Because the Wnt signaling pathway plays a role in many organ systems throughout the body, our studies are important not only to understand kidney development. , but also to obtain relevant information on the development of other organs.
“Additionally, it has been an honor to work in the McMahon Lab and I am grateful to the people I have met along the way.”
More information:
Helena Bugacov et al, Dose-dependent responses to canonical Wnt transcriptional complexes in the regulation of mammalian nephron progenitors, Development (2024). DOI: 10.1242/dev.202279
Balint Der et al, Cadherin adhesion complexes direct cell aggregation in Wnt-induced epithelial transition of nephron progenitor cells, Development (2024). DOI: 10.1242/dev.202303
Provided by the Keck School of Medicine of USC
Quote: Stem cell studies detail how progenitor cells self-renew, differentiate, and aggregate into early kidney structures (October 1, 2024) retrieved October 1, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.