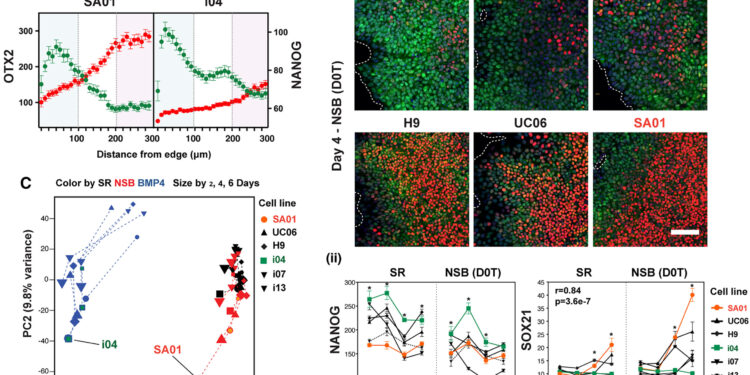
Cell lineage variation in the emergence of neural fate from pluripotency (A) Spatial expression at day 6 under SR condition in SA01 and i04 lines. (B) Variation in NANOG and SOX21 expression in PSC lines. (i) Representative images. Scale bar, 100 μm. (ii) Expression levels in each lineage over time (n = 5, independent experiments; ∗Comparison between SA01 and i04: p < 0.001; r and p (C) PCA showing the differentiation trajectories. Credit: Stem Cell Reports (2024). DOI: 10.1016/j.stemcr.2024.07.004
Researchers at Johns Hopkins University School of Medicine have mapped variations in human stem cells, explaining how an individual’s cells can shape a unique “developmental dance” at the molecular level, controlling the creation of the brain and body. These findings provide insights into the origins and implications of cellular variation in individuals and could advance the design of individualized therapies and methods for rebuilding diseased or damaged human organs.
The study, published on September 10 in Stem Cell Reportsused human induced pluripotent stem cells, a type of stem cell equivalent to cells in early embryonic development that can develop into multiple tissue types. Studying pluripotent cells from multiple donors showed how genetic and epigenetic (non-nuclear DNA) factors contribute to individual variation in early brain development.
“Every human body is created from a single cell, and when the cells of the developing embryo begin to divide, they perform a genetically programmed choreography to build the new human being,” says Carlo Colantuoni, Ph.D., assistant professor in the departments of neurology and neuroscience at the Johns Hopkins University School of Medicine.
But, Colantuoni notes, while this developmental dance has major milestones that are consistent across the human population (and the animal kingdom), this study uncovered molecular patterns and gene expression traits that alter the conserved human milestones and create unique individual development.
In this study, researchers from Johns Hopkins University and Yale University used a process known as “cellular reprogramming,” in which mature cells can revert to their embryonic pluripotent state, a technique that won a Nobel Prize in 2012. Specifically, the researchers took skin cells from adults and reverted them to an early embryonic state. Called induced pluripotent stem cells (iPSCs), they have the potential to develop into any cell in the human body.
The researchers allowed the iPS cells to grow and interact without restraint, allowing the cells to reveal their inherent nature and individuality. RNA sequencing, which looks at how much RNA each gene makes, gave the researchers a detailed molecular perspective on what the cells were doing.
By then combining RNA data from induced pluripotent stem cells (iPSCs) with data from developing mouse embryos, the researchers were able to map how donor cells vary across the mammalian developmental landscape. This revealed systematic differences in how individual human cells evolve during early development, differences that were also observed in publicly available data from 100 human stem cell donors.
The researchers observed two types of patterns, or rhythms, of the developmental dance emerging from the RNA data. The first type of pattern was consistent across individual donors, defining large modules responsible for creating parts of the human body such as the brain, heart, and liver. The second type of pattern was more subtle, more evolutionarily recent, and present only in cells from specific individuals, regardless of the cells’ ultimate function.
Interestingly, the new patterns showing individual variations in human programmed choreography were present not only in cells observed in the lab, but also in mature cells in the donors’ bodies. The researchers say this indicates that the variations observed in cell development, each specific to an individual, influenced the steps the donor cells took during early development.
By logical extension, the scientists explain, their findings not only indicate the unique steps an individual’s cells take, but also that those cells hold information about a person’s health or disease risk over the course of their lifetime. Colantuoni says the findings could pave the way for more precise, individualized approaches to regenerative medicine.
“We advocate the study of induced pluripotent stem cells and their derivatives to understand each patient’s risk for developing a complex disease and the specific treatments that will be most effective for that individual,” Colantuoni says. “We envision a future in which a patient’s traditional medical history, genome sequence, and living cells will be routinely used together to diagnose and personalize patient treatment.”
Colantuoni says scientists are just beginning to uncover the specific molecular mechanisms underlying differences in human cell development. Further studies in larger and more diverse groups of donors are needed to understand the long-term health effects of an individual’s developing cells evolving to the beat of their own drum.
Other scientists who contributed to this research include: Suel-Kee Kim, Seungmae Seo, Genevieve Stein-O’Brien, Amritha Jaishankar, Kazuya Ogawa, Nicola Micali, Yanhong Wang, Thomas Hyde, Joel Kleinman, Elana Fertig, Joo Heon Shin, Daniel Weinberger, Joshua Chenoweth, Daniel Hoeppner, and Ronald McKay.
More information:
Suel-Kee Kim et al., Individual variation in the emergence of anterior-posterior neuronal fates of human pluripotent stem cells, Stem Cell Reports (2024). DOI: 10.1016/j.stemcr.2024.07.004
Provided by Johns Hopkins University School of Medicine
Quote: Stem cell map reveals molecular choreography behind individual variation in human development (2024, September 25) retrieved September 25, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.