Credit: Advanced Materials Science and Technology (2023). DOI: 10.1080/14686996.2023.2170164
Vaccines and therapies based on messenger RNA could be more easily administered thanks to a non-toxic polymer that protects the RNA and controls its release inside cells.
The advent of vaccines using messenger RNA (mRNA) to direct the synthesis of immunogenic proteins, best known in COVID-19 vaccines, is prompting researchers to find better ways to keep mRNA stable and administer effectively.
A team from the University of Tokyo, with collaborators in Japan and China, has developed polymers capable of interacting with, stabilizing and enveloping mRNA, enabling highly efficient delivery into cultured human cells and live mouse cells. They published their work in the journal Advanced Materials Science and Technology.
“Beyond vaccines against infectious diseases, mRNA presents promising avenues for unprecedented treatments such as protein replacement therapies, gene editing and immunotherapies,” explains Horacio Cabral of the team at the University of Tokyo. “But to unlock the full potential of these advanced therapies, the development of safe and efficient transport systems is paramount.”
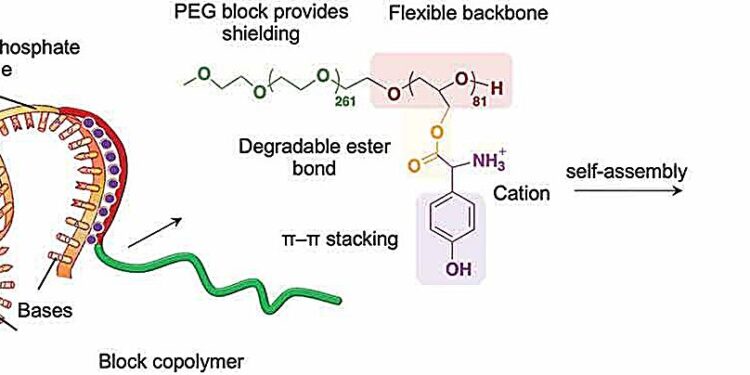
The researchers investigated ways to fine-tune the structure of their polymer molecules to allow them to interact with mRNA to protect it. The biocompatible, nontoxic polymers were of a type called block copolymers, constructed from alternating segments of different chemical groups, in this case polyethylene glycol and polyglycerol.
But the key to achieving proper interaction with mRNA was attaching specific positively charged groups of amino acids to the long polymer backbone. The positive charge generally attracts the polymer toward a negatively charged RNA, and the chosen amino acids were also able to interact with parts of the mRNA in a process called pi–pi (π–π) stacking. This involves interactions between electrons in a feature called pi bonds in cyclic molecular rings stacked side by side in the interacting molecules.
“This is a highly customizable approach, allowing us to fine-tune the interactions of our polymer with mRNA,” explains Cabral. As a result, the mRNA was stabilized very efficiently, overcoming a major drawback of instability encountered with alternative approaches.
The polymer and mRNA spontaneously assembled into spherical bundles – micelles – that efficiently delivered the mRNA cargo into cultured cells as well as into mouse cells after intramuscular injection. The mRNA was readily released inside cells to generate the proteins it encoded with high efficiency and for a significantly longer duration than alternative approaches.
“This work was very difficult due to the delicate nature of mRNA, a very fragile molecule that requires protection outside the target cells but immediate exposure to the cellular machinery once inside,” explains Cabral . He adds: “Our success is exciting because of its potential to transform mRNA delivery technologies, enabling precise engineering, innovative delivery strategies and overcoming critical obstacles to improve the stability and efficacy of therapies. based on mRNA. »
More information:
Wenqian Yang et al, Blocked catiomers with flanking hydrolyzable tyrosinate groups enhance in vivo mRNA delivery via π–π stacking-assisted micellar assembly, Advanced Materials Science and Technology (2023). DOI: 10.1080/14686996.2023.2170164
Provided by the National Institute of Materials Science
Quote: Stabilization of mRNA vaccines for delivery to cells (February 16, 2024) retrieved February 16, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.