Credit: Cell Reports (2024). DOI: 10.1016/j.celrep.2023.113644
Breast cancer is the most common cancer in women. The development of breast cancer often originates from the epithelial cells of the mammary gland, the very cells that specialize in milk production during and after pregnancy.
A team of researchers from Friedrich Schiller University Jena (Germany), Shenzhen University (China) and Jena University Hospital (Germany) took a closer look at this specialization process and deciphered a mechanism molecular which also appears to play an important role in cancer development.
It may be possible to develop new diagnostic procedures and treatment methods for breast cancer based on these research results now published in Cell Reports.
Cellular differentiation, i.e. cell specialization, is the process by which cells take on different tasks. During lactogenesis – the hormone-triggered process that allows the mammary glands to produce milk – the affected cells multiply first. The proteins needed for this are produced by ribosomes. Ribosomal RNA, or rRNA for short, is a fundamental building block of ribosomes.
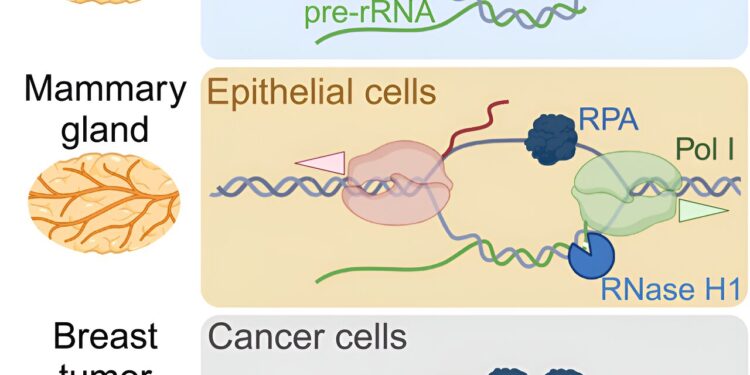
If more proteins are needed, the demand for rRNA also increases and its synthesis in the cell nucleus accelerates accordingly. At the end of lactogenesis, specialized cells stop growing and reduce rRNA synthesis again. This regulatory mechanism takes place at the epigenetic level, which means that it is not the DNA itself that changes, but its packaging, for which another type of RNA is responsible.
“We discovered that a long non-coding RNA called PAPAS, which I discovered a few years ago, acts on DNA packaging and reduces the production of rRNA,” explains Dr. Holger Bierhoff, who leads the project. at the University of Jena.
“More precisely, PAPAS influences access to active regions of DNA and determines whether or not these are copied into RNA. If a lot of ribosomes and proteins, and therefore a lot of rRNA, are needed, the PAPAS synthesis is reduced. If this process needs to be stopped, the PAPAS level is increased.
The Jena experts also discovered that PAPAS not only plays an important role in cell proliferation, but also in specialization. “When we deactivated PAPAS by genetic manipulation in the cells, we observed that the development of milk-producing cells no longer functioned properly,” explains Bierhoff.
High PAPAS level, low tumor growth
The synthesis of rRNA is also increased in cancer cells, because they multiply quickly and require a lot of protein, and therefore a lot of ribosomes. “We therefore asked ourselves whether the regulatory mechanism we observed also plays a role in the development of breast cancer. The answer is clearly yes,” explains cell biologist Bierhoff.
“When we reduced PAPAS synthesis and turned off cell specialization, we observed that the cells developed the characteristics of cancer cells.” On the other hand, researchers have shown both in cell cultures and in mice that a high level of PAPAS reduces tumor growth as well as the spread of metastases.
But how do cancer cells manage to stop the production of PAPAS and thus stimulate the synthesis of rRNA? “We also found a mechanism for this,” says Bierhoff. “The production of PAPAS requires a molecular signal at the beginning of the PAPAS gene. This signaling structure is regulated by particular proteins, which can resolve or block the structure. We observed that the production of these proteins is particularly high in cancer cells of the breast. The more aggressive the tumor, the more they are present.”
For Holger Bierhoff, the research results are promising in two respects. “First of all, we see that PAPAS can be an interesting marker to assess the aggressiveness of a breast tumor. This information could potentially be used as a diagnostic tool,” he explains. “Second, we are already working on the development of RNA therapy for the treatment of cancer. We know the mechanism by which PAPAS regulates rRNA synthesis and we know which region of RNA is required for this.
“Now the idea is to artificially produce this part of PAPAS, package it in nanoparticles and introduce it into cancer cells, thereby restoring its function. In this way, we would reduce the synthesis of rRNA, the cancer needs to proliferate.” This strategy would be similar to mRNA vaccines, such as the one against COVID-19, however, here a regulatory RNA instead of a protein-coding RNA would be applied.
More information:
Sijia Ren et al, PAPAS promotes mammary epithelial cell differentiation and suppresses breast carcinogenesis, Cell Reports (2024). DOI: 10.1016/j.celrep.2023.113644
Provided by Friedrich Schiller University Jena
Quote: Special RNA shown to suppress breast cancer cell formation (January 16, 2024) retrieved January 16, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.