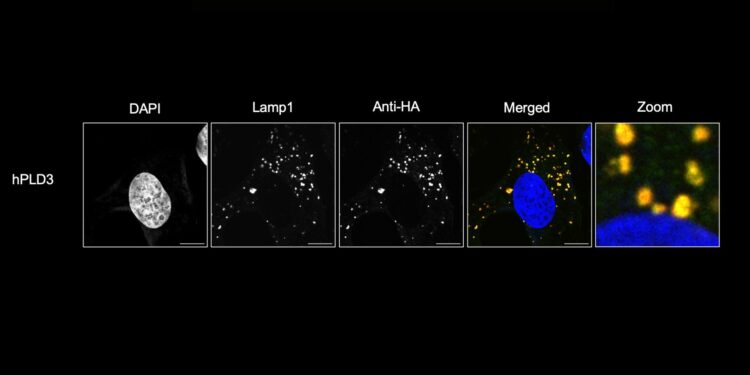
PLD3 is localized in the lysosomes of HMC3 microglial cells, a population of immune cells that play a key role in maintaining brain health. Credit: The Farese & Walther laboratory
Scientists have gained a better understanding of a molecule that regulates lipid levels in the brain. This breakthrough could eventually lead to treatments for diseases like frontotemporal dementia and Alzheimer’s disease. The results are published in the journal Cell.
How can we produce a fat molecule that participates in the breakdown of other fats in the brain, without being destroyed itself? This is a question that has preoccupied scientists for half a century.
BMP, or bis(monoacylglycerol)phosphate, is a phospholipid located in lysosomes, the trash bins of the cell.
“BMP is a degradation cofactor, but it itself is very, very stable and has unusual chemistry,” says Tobias Walther, a researcher at the Howard Hughes Medical Institute. “As a result, no one knew how it was made.”
In the new study, the team of Walther and Robert Farese, Jr. of the Cell Biology Program at the Sloan Kettering Institute report that two enzymes, phospholipases D3 and D4 (PLD3 and PLD4), are also required to make BMP in laboratory tests. as in human cells and animal models.
For more than 15 years, Walther and Farese’s laboratory has studied frontotemporal dementia (FTD), the disease with which actor Bruce Willis was diagnosed in 2023. It affects both the frontal and temporal lobes of the brain, responsible of personality, judgment, and speech. FTD is the most common cause of dementia in people under the age of 60 and there is no known cure or treatment.
In previous work, researchers found that FTD patients had elevated levels of gangliosides, a type of lipid linked to a sugar, in their brains. It turned out that these molecules were accumulating due to a degradation problem.
“That’s when we really got interested in this BMP molecule and found that it was extremely low in FTD brains,” says Farese. High levels of gangliosides are toxic and changes in BMP activity are associated with neurodegenerative diseases, suggesting that controlling ganglioside amounts is important for proper brain function.
Mirror, mirror on the wall
As molecules, BMP is special, Walther explains. “The molecules have a pattern that looks like a left hand or a right hand that is the same at one level, but one is a mirror image of the other,” he says. Lipids and phospholipids are almost always in the “R” configuration, but BMP is one of the few phospholipids to occur in the opposite form, called “S.” In fact, “disability” can appear in two places in BMP, and both are in the S form.
The S nature of BMP is what makes it so stable in the lysosome, when all other lipids, which are R, are destroyed. But the 50-year-old question is: If lipids are R, how do any of them become S?
Changing the handling of a molecule is no small feat and happens rarely, says Shubham Singh, a postdoctoral researcher at the Sloan Kettering Institute who led the study. “Everything in lipid biochemistry starts from a molecule called glycerol 3-phosphate, and that’s R,” Singh explains. “So, at what step do you convert R to S, or right hand to left hand, to create BMP?”
Exchange meeting
Singh and his colleagues observed that human cells exchanged a glycerol between two different molecules to produce the S form of BMP in a reaction called transphosphatidylation. Next, examining protein sequences for enzymes that might interact with lipids, Singh decided to test for phospholipase D enzymes.
Through a series of experiments, the researchers concluded that PLD3 and PLD4 catalyze the reaction. Increasing expression of PLD3 or PLD4 increased BMP levels, and mutations that abolished their activity resulted in decreased BMP levels.
Interestingly, PLD3 mutations that cause spinocerebellar ataxia 46, a rare neurodegenerative disease, or increase the risk of Alzheimer’s, also reduce BMP synthesis. Similar results on brain lipids were obtained when PLD3 was knocked out in mice.
“The paper’s findings that these two related enzymes, PLD3 and PLD4, produce BMP complete an important piece of the BMP puzzle, and these enzymes do so in an elegant way that results in a reversal of stereochemistry, or hand, of parts of the molecule system,” says Jeremy Baskin, a cell biologist at Cornell University, who was not involved in the work.
Baskin adds that the study broadens the understanding of the PLD3 and PLD4 domain, because unlike other members of the phospholipase D class, the functions of these two enzymes were not well understood. In fact, he says PLD3 and PLD4 were once thought to only degrade nucleic acids, but now appear to play a new role in making a lipid. Walther said that was just one of the surprising results.
“We were also surprised because other people had reported that another enzyme could make BMP,” he says. This enzyme could produce BMP, but it was not the correct stereochemical form.
Now that the team knows more about a crucial step in BMP synthesis, they are studying the role of these lipids in other neurodegenerative diseases. And although they have not yet considered therapies based on their findings, it is possible that such approaches could help patients in the future.
Ultimately, Walther says, this work demonstrates the value of basic research. “It really took us sitting down and charting paths with perseverance and a little bit of serendipity to pursue this,” he says. “There remain so many unturned stones and fundamental discoveries to be made.”
More information:
Shubham Singh et al, PLD3 and PLD4 synthesize S,S-BMP, a key phospholipid enabling lipid degradation in lysosomes, Cell (2024). DOI: 10.1016/j.cell.2024.09.036
Cell
Provided by the Howard Hughes Medical Institute
Quote: Solving a 50-year-old mystery could lead to treatments for neurodegenerative diseases (October 18, 2024) retrieved October 18, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.