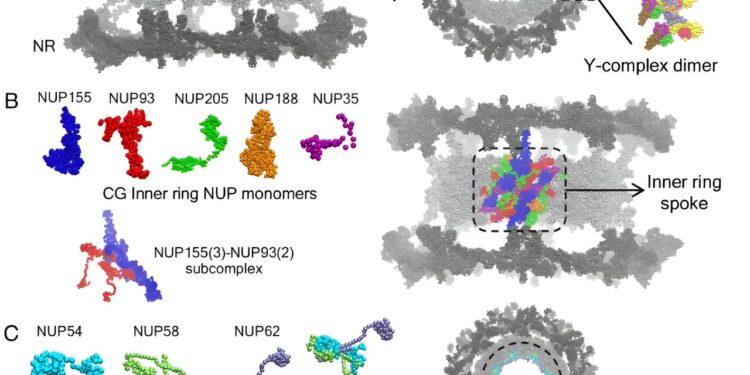
Overview of the CG molecular model of CR, NR and IR. (A) The upper panel shows the CG molecular model of each Y-complex NUP monomer. A side and top view of the NPC highlighting a single copy of the Y-complex dimer is shown in the lower panel. The rest of the NPC is represented by gray spheres. The internal and external Y complex monomer is labeled in the Y complex dimer. (B) The CG molecular model of each NUP monomer in the IR is shown in the upper left panel. The NUP155–NUP93 subcomplex composed of 3 copies of NUP155 and 2 copies of NUP93 is shown in the lower left panel. A single unit of the IR ray is shown (right) in the composite CG model of the NPC and is highlighted in color. (C) The CG molecular model of each FG NUP monomer and the NUP54–NUP58–NUP62 heterotrimeric subcomplex is shown. A top view of the NPC is shown, highlighting the NUP NUP62 FGs lining the central channel. (D) In the left panel, the membrane-binding β-propeller domains of NUP160, NUP133, and NUP155 are highlighted. The rest of the CG protein beads (no attractive interactions with lipids) are shown as gray spheres. In the right panel, the membrane-embedded composite dilated NPC model is shown. In the inset, 4-site CG lipids are shown. The CG lipid headgroup is represented by pink spheres. The interfacial CG bead and the two tail beads of the lipid CG are shown in white spheres. Credit: Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2313737121
Because viruses have to hijack someone else’s cell in order to replicate, they have become very good at this and have invented all sorts of tricks.
A new study by two University of Chicago scientists has revealed how HIV sneaks into the nucleus when it invades a cell. The work is published in the journal Proceedings of the National Academy of Sciences.
According to their models, the HIV capsid, which is cone-shaped, points its smaller end into the pores of the nucleus and then sinks there. Once the pore is open enough, the capsid is elastic enough to squeeze through. According to the scientists, it is important to note that the structural flexibility of the capsid and the pore itself play a role in the infiltration process.
This discovery, resulting from a simulation of thousands of interacting proteins, will pave the way for a better understanding of HIV and suggest new targets for therapeutic drugs. “For example, you could try to make the HIV capsid less elastic, which our data suggests would hinder its ability to get inside the nucleus,” said Arpa Hudait, a research scientist at UChicago and first author of the study. ‘article.
The study also provides the most comprehensive simulation to date of the nuclear pore itself, which plays an important role in many biological processes.
Capsid versus cell
Hudait is a member of the laboratory of Gregory Voth, the Haig P. Papazian Professor of Chemistry, who specializes in simulations aimed at unraveling the complex biological processes that occur when viruses attack a cell.
In this case, Voth and Hudait focused on what is known as the HIV capsid, the capsule containing HIV’s genetic material, which enters the nucleus of a host cell and forces the cell to make copies of the key components of HIV.
The capsid is a complex piece of machinery, composed of more than a thousand proteins assembled in the shape of a cone, with an increasingly large end. To penetrate the nucleus of the host cell, it must squeeze through. But scientists didn’t know exactly how this happens.
“This part remained a mystery for years,” said Voth, the paper’s lead author. “For a long time, no one knew whether the capsid breaks before entering the pore or after, for example.”
Recent imaging studies suggest that the capsid remains intact and wriggles through the nuclear pore complex. This is essentially the mail slot where the core sends and receives deliveries.
“The pore complex is an incredible machine; it can’t let just anything into the nucleus of your cell, otherwise you’d have serious problems, but it has to let quite a few things in. And somehow on the other, the HIV capsid has figured out how to infiltrate,” Voth said. “The problem is we can’t watch it live. It takes heroic experimental efforts to get even a single snapshot.”
To fill these gaps, Hudait built a careful computer simulation of the HIV capsid and nuclear pore complex, representing thousands of proteins working together.
By running the simulations, the scientists found that it was much easier for the capsid to enter the pore by first trapping its smaller end and then gradually inserting itself into it. “It doesn’t need active work to do it, it’s just physics — what we call an electrostatic ratchet,” Voth said. “It’s kind of like you’ve ever had a tight seat belt on you, where it just keeps getting tighter.”
They also found that the pores and capsid deformed over time. Interestingly, the network of molecules that make up the capsid structure develops small, lower-order regions to accommodate pressure stress. “This is not a strong squeeze or expansion, as one might have expected,” Hudait said.
This finding could help explain why capsids have a conical rather than cylindrical shape, which may at first seem easier to slip through a pore.
Scientists said every detail of HIV’s journey through the body is an opportunity to discover vulnerabilities that drugs could be developed to target. It is also a broad look at a fundamental aspect of biology.
“I think this modeling also gives us a new way of understanding how many things go into the nucleus, not just HIV,” Voth said.
The simulations were performed at the Texas Advanced Computing Center at the University of Texas at Austin and the Research Computing Center at UChicago.
More information:
Arpa Hudait et al, HIV-1 capsid shape, orientation, and entropic elasticity regulate translocation in the nuclear pore complex, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2313737121
Provided by the University of Chicago
Quote: Simulations show how HIV infiltrates the nucleus of the cell (January 25, 2024) retrieved January 25, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.