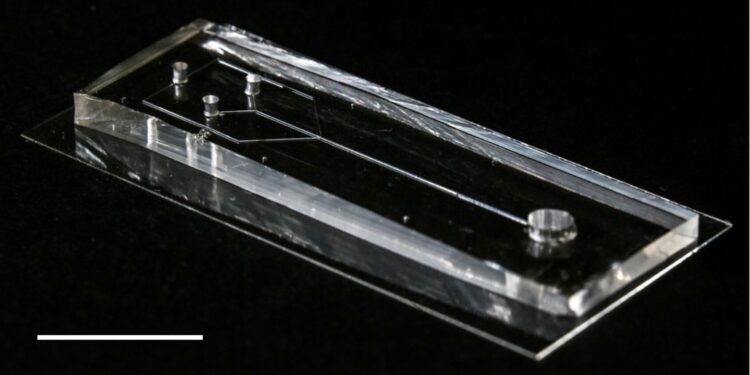
The microfluidic device. The spidroin precursor solution is placed at one end and then pulled to the other end using negative pressure. As spirroins flow through microfluidic channels, they are exposed to precise changes in the chemical and physical environment and self-assemble into silk fibers. Credit: RIKEN
Researchers have successfully created a device that weaves artificial spider silk that closely matches what spiders produce naturally. The artificial silk gland was able to recreate the complex molecular structure of silk by mimicking the various chemical and physical changes that occur naturally in a spider’s silk gland.
This environmentally friendly innovation is a big step towards sustainability and could impact several industries. The study, led by Keiji Numata of the RIKEN Center for Sustainable Resource Science in Japan, with colleagues from the RIKEN Pioneering Research Cluster, was published in the journal Natural communications.
Famous for its strength, flexibility and lightness, spider silk has tensile strength comparable to steel of the same diameter and an unprecedented strength-to-weight ratio. Additionally, it is biocompatible, meaning it can be used in medical applications, as well as biodegradable. So why isn’t everything made of spider silk? Large-scale harvesting of spider silk has proven impractical for several reasons, leaving scientists to develop a way to produce it in the laboratory.
Spider silk is a biopolymeric fiber made from large proteins with highly repetitive sequences, called spirroins. Inside the silk fibers are molecular substructures called beta sheets, which must be properly aligned for the silk fibers to possess their unique mechanical properties. Recreating this complex molecular architecture has baffled scientists for years. Rather than trying to design the process from scratch, RIKEN scientists took a biomimicry approach.
As Numata explains: “In this study, we attempted to mimic the natural production of spider silk using microfluidics, which involves the flow and manipulation of small amounts of fluids through narrow channels. Indeed, one could say that the spider’s silk gland functions as a kind of natural microfluidic device.
The device developed by the researchers looks like a small rectangular box in which tiny channels are dug. The spidroin precursor solution is placed at one end and then pulled to the other end using negative pressure.
As spirdroins flow through microfluidic channels, they are exposed to precise changes in the chemical and physical environment, made possible by the design of the microfluidic system. Under appropriate conditions, proteins self-assemble into silk fibers with their characteristic complex structure.
The researchers experimented to find these correct conditions and were ultimately able to optimize the interactions between different regions of the microfluidic system. Among other things, they discovered that using force to push proteins through didn’t work; Only when they used negative pressure to extract the spidroin solution could continuous silk fibers with the correct alignment of the beta sheets be assembled.
“It was surprising how robust the microfluidic system was, once the different conditions were established and optimized,” says lead scientist Ali Malay, one of the paper’s co-authors. “The assembly of the fibers was spontaneous, extremely rapid and highly reproducible. Importantly, the fibers exhibited the distinct hierarchical structure found in natural silk fibers.”
The ability to artificially produce silk fibers using this method could provide many benefits. Not only could this help reduce the negative impact of current textile manufacturing on the environment, but the biodegradable and biocompatible nature of spider silk makes it ideal for biomedical applications, such as artificial sutures and ligaments.
“Ideally we want to make a real impact,” says Numata. “For this to happen, we will need to expand our fiber production methodology and make it a continuous process. We will also evaluate the quality of our artificial spider silk using multiple parameters and make further improvements based on there.”
More information:
Jianming Chen et al, Replication of spider silk self-assembly by shearing using microfluidics, Natural communications (2024). DOI: 10.1038/s41467-024-44733-1
Quote: Scientists spin naturalistic silk from artificial spider gland (January 22, 2024) retrieved January 22, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.