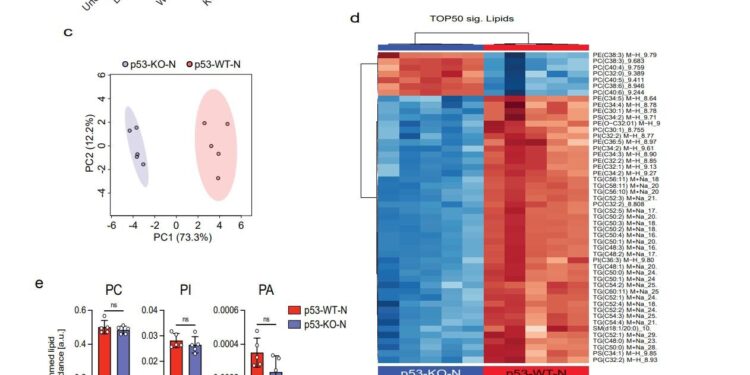
a, Quantification of lipid droplets by Oil-Red-O staining in undifferentiated 3T3-L1 and 3T3-L1 induced to differentiate in the presence of regular differentiation medium or differentiation medium supplemented (1:1) with CM control (WT) or p53KO WEP cells treated for 24 hours with or without 7μM Nutlin-3a before harvesting. Each point represents ImageJ’s quantification of a non-overlapping microscopic field (5 randomly chosen fields for each condition). b, Number of lipid molecular species per lipid subclass identified in 3T3-L1 cells in all samples. c, Principal component analysis (PCA) of lipidomic data from 3T3-L1 cells differentiated in the presence of CM from WT or p53KO WEP cells treated with Nutlin-3a. d, Heatmap showing the top 50 lipids significantly different in cells in (c). The heat map was constructed using a two-sample t-test (p-value <0.05). Individual lipids are presented in rows and samples are displayed in columns, organized by clustering analysis (clustering distance was calculated by Pearson and clustering algorithm estimated by Ward). e, summed lipid abundance of membrane phospholipids detected in 3T3-L1 cells in (c). f, summed lipid abundance of lysophospholipids detected in 3T3-L1 cells as in (c). g, Total lipid abundance of membrane phospholipids containing polyunsaturated fatty acids (PUFAs) in 3T3-L1 cells in (c). Abbreviations: phosphatidylcholine (PC); phosphatidylinositol (PI); Phosphatidic acid (PA); Lysophosphatidylethanolamine (LPE); Lysophosphatidylglycerol (LPG); Lysophosphatidylinositol (LPI); Example data (n = 5) are expressed and samples are displayed in columns, organized by clustering analysis (clustering distance was calculated by Pearson and the clustering algorithm was polyunsaturated mean ± standard deviation; unpaired two-sample t-test. Credit: Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2311460120
Mutations in the tumor suppressor p53 not only have a growth-promoting effect on cancer cells themselves, but also influence cells in the tumor microenvironment. Scientists from the German Cancer Research Center (DKFZ) and the Weizmann Institute in Israel have shown that p53-mutated mouse breast cancer cells reprogram fat cells. The engineered fat cells create an inflammatory microenvironment, impairing the immune response against the tumor and thereby promoting cancer growth.
No other gene is mutated as frequently in human tumors as the p53 tumor suppressor gene. In about 30 percent of all cases of breast cancer, the cancer cells have mutations or losses of the p53 gene. These mutations limit the ability of p53 to act as a “cancer brake” of p53 and prevent the development and progression of cancer.
The effects of p53 mutations on cancer cells have been extensively researched. However, the understanding that p53 mutations in cancer cells can also affect cells in the tumor microenvironment – and thus stimulate cancer growth – is only progressing slowly.
A team of researchers led by Almut Schulze of the DKZF and Moshe Oren of the Weizmann Institute in Israel studied the effects of p53 mutations in breast cancer cells on fat cells, called adipocytes. As breast cancer progresses, adipocytes, one of the main cell types in breast tissue, undergo transformation. Research results indicate that this increases the aggressiveness and resistance to treatment of surrounding breast cancer cells.
The team of Schulze and Oren demonstrated this in adipocytes from mouse breast tissue: the carcinogenic properties of adipocytes are potentiated when breast cancer cells carry p53 mutations.
The researchers treated immature adipocytes with a culture medium in which breast cancer cells with or without p53 mutations had previously grown. This treatment triggered profound changes in metabolism and gene activity in adipocytes and increased the production of pro-inflammatory messengers.
Maturation of adipocytes was prevented, while mature adipose cells were reverted to an immature stage. These effects were only slight after treatment with cell culture medium from breast cancer cells with functional p53, but were very evident in the case of medium from cancer cells with mutated p53.
The researchers then transferred breast cancer cells with mutated or functional p53 as well as pretreated fat cells to mice and compared the resulting tumors. If p53 was mutated in cancer cells, the number of immunosuppressive myeloid cells in the tumor increased. Migrated immune cells carried more PD-L1 on their surface, which acts as a powerful brake on tumor immune defense.
A particularly surprising result was that breast cancer cells with certain p53 mutations were able to reprogram neighboring precursor fat cells, directly or indirectly, to be even more pro-inflammatory than breast cancer cells that had completely lost the p53 tumor suppressor.
“P53 defects in breast cancer cells appear to be the central driver of tumor-promoting fat cell reprogramming,” says Schulze, who led the study with Oren. “Adipose cells are an essential component of breast tissue and may therefore have a considerable influence on tumor progression. A detailed understanding of the interaction between p53-mutated cancer cells and adipocytes could therefore provide new clues as to how The progression of breast cancer can be stopped.”
The study is published in the journal Proceedings of the National Academy of Sciences.
More information:
Ori Hassin et al, p53-deficient breast cancer cells reprogram preadipocytes towards immunomodulatory cells protective against tumors, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2311460120
Provided by the German Cancer Research Center
Quote: Scientists show reprogrammed fat cells support tumor growth (January 3, 2024) retrieved January 3, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.