The crystalline structure of newly discovered Zangenite. Credit: Shihao Zang / Nyu
Crystals – sugar and salt table with snowflakes and diamonds – do not always grow in a simple way. Researchers from New York University have captured this trip of amorphous Blob to the ordered structures in a new study published in Nature communications.
By exploring how the crystals are formed, the researchers also came across an unusual rod crystal that had never been identified before, appointing it “Zangenite” for the graduate student of Nyu who discovered it.
Chaos order
Crystals are solid materials composed of particles that are organized in repetitive patterns. This self -assembly process – “orchestrate the order of chaos”, as researchers describe it – was once thought to follow a predictable and classic growth scheme. But instead of always forming a construction element by a building block, scientists learn that crystals can develop through more complex ways.
To study the formation of crystals, some researchers use crystals made up of small spheres called colloidal particles, which are tiny but much larger than the atoms that make up other crystals.
“The advantage of studying colloidal particles is that we can observe the crystallization processes at a unique level, which is very difficult to do with atoms because they are too small and fast. With colloids, we can look at the crystals formed with our microscope,” said Stefano Sacanna, professor of chemistry in NYU.
A two -step process
To shed light on how colloidal crystals are formed, the researchers have conducted experiences to carefully observe how loaded colloidal particles behave in different conditions of growth while they pass from salt water suspensions to fully formed crystals. The team also directed thousands of computer simulations – led by Glen Hocky, assistant chemistry professor in Nyu – to model how crystals develop and help explain what they have observed in experiences.
Researchers have determined that colloidal crystals are formed through a two -step process: particle amorphous spots condense first before turning into ordered crystal structures, resulting into a diversified range of types of crystals and shapes.
An unexpected find
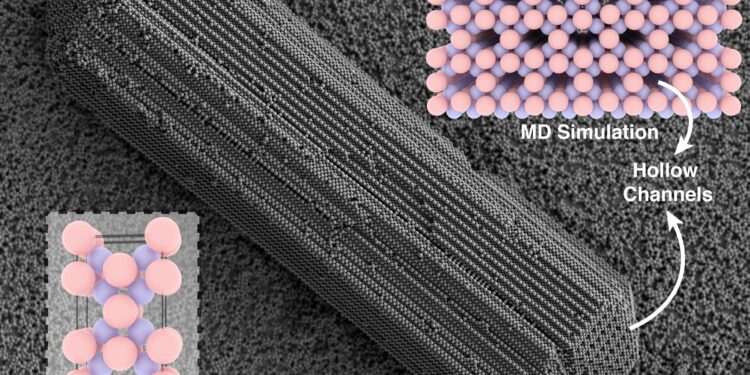
During these experiences, Ph.D. Student Shihao Zang came across a rod -shaped crystal that he could not identify. With the naked eye, it looked like a crystal discovered previously in the laboratory, but the exam more closely, the combination of particles was different and the tips of this crystal contained hollow canals. Zang compared the unknown structure with more than a thousand crystals found in the natural world and has still not found a match.
By turning to Hocky’s computer modeling, the researchers simulated a crystal which was exactly the same, allowing them to study its hollow shape elongated with even more details.
“This was confusing because generally the crystals are dense, but it had empty channels that worked on the length of the crystal,” said Hocky, who is also a member of the faculty of the Simons Center for Computational Physical Chemistry in Nyu.
“Thanks to this synergy of experiences and simulation, we realized that this crystalline structure had never been observed before,” added Sacanna.
They named the newly discovered Crystal L3S4 on the basis of its composition, but began to call it informally “zangenite” during laboratory meetings, since Zang discovered it. The name is stuck.
“We are studying colloidal crystals to imitate the real world of atomic crystals, but we have never imagined that we would discover a crystal that we cannot find in the real world,” said Zang.
-
Nyu Ph.D. Student Shihao Zang. Credit: David Song / Nyu
-
Instead of always forming a construction element by a building block, scientists learn that crystals can develop through more complex ways. Credit: Shihao Zang / Nyu
The discovery of the zangenite creates an opportunity to explore uses of hollow and low density crystals and can open the way to the search for new additional crystals.
“The channels inside the zangenite are similar to the characteristics in other materials which are useful for filtering or locking things inside,” said Hocky.
“Before, we thought it would be rare to observe a new crystalline structure, but we may discover new additional structures that have not yet been characterized,” said Sacanna.
More broadly, a more in -depth understanding of the way in which crystals are formed are promising for the development of new materials, including photonics band materials which are fundamental for lasers, fiber optic cables, solar panels and other technologies that transmit or harvest light.
The other authors of the study are Sanjib Paul, Cheuk Leung, Michael Chen and Theodore Hueckel.
More information:
Shihao Zang et al, direct observation and control of non -classic crystallization ways in binary colloidal systems, Nature communications (2025). DOI: 10.1038 / S41467-025-58959-0
Provided by New York University
Quote: Scientists observe how blobs form crystals and discover a new type of crystal (2025, April 28) recovered on April 28, 2025 from
This document is subject to copyright. In addition to any fair program for private or research purposes, no part can be reproduced without written authorization. The content is provided only for information purposes.