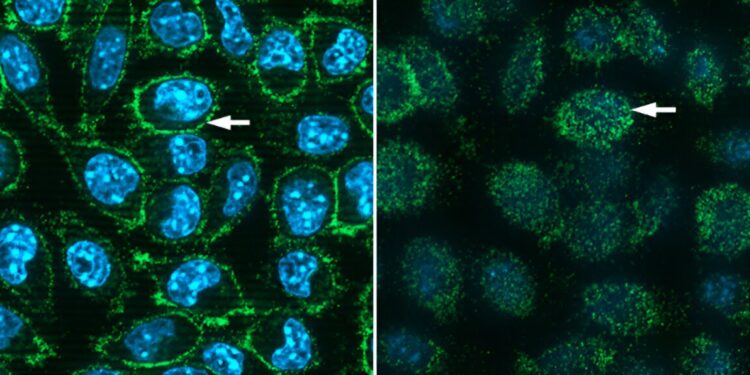
The scientists used fluorescence microscopy to visualize human ACE2 proteins expressed on mouse cells after incubating the cells with fluorescently labeled SARS-CoV-2 spike protein (green). Left: View in the middle of the cells with the arrow pointing towards the cell membrane. Right: a view of the membrane surface from outside the cells. Blue fluorescence corresponds to DNA present in cell nuclei. Angela Kim of NASA’s Space Radiation Laboratory User Support Group provided expert assistance with the microscopy. Credit: Brookhaven National Laboratory
A team of scientists from the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory and Columbia University have demonstrated a way to produce large quantities of the receptor that SARS-CoV-2, the virus that causes COVID -19, binds to the surface. of human cells. This binding between the now infamous viral spike protein and the human “ACE2” receptor is the first step in virus infection. Producing functional human ACE2 proteins in mouse cells provides scientists with a new way to study and potentially use these receptors. Furthermore, as described in an article just published in the journal Virologythe method could facilitate the study of other complex proteins that have proven difficult to produce by other means.
The initial goal of Brookhaven scientists early in the pandemic was to make large quantities of human ACE2 and then bind the protein to nanoparticles. The ACE2-coated nanoparticles could then be tested as antiviral therapeutic agents and/or sensors to detect viral particles.
“For either of these applications, you need large amounts of protein, and the protein has to be fully functional,” said Paul Freimuth, a virologist at Brookhaven Lab who led the research in collaboration with scientists. scientists at the Center for Functional Nanomaterials (CFN) at Brookhaven Lab. “But making functional membrane proteins like ACE2 is particularly difficult because the process by which proteins are localized to the cell membrane is complex.”
One reason is that these proteins are modified in various ways after they are synthesized and before they are inserted into the cell membrane. In particular, carbohydrate molecules added to proteins play a key role in both how the long protein chain folds into its final 3D structure and how the protein functions in the membrane.
“Carbohydrates make up about a third of the mass of ACE2 proteins,” Freimuth said.
The simplest cells that scientists use to artificially generate proteins, namely bacteria, lack the enzymes needed to fix these extra carbohydrates. So the Brookhaven team turned to mouse cells, which, as mammals, are more like us and are therefore capable of carrying out the same type of carbohydrate processing. Mouse cells are known to be able to sense and express “foreign” genes. And although mouse cells also make an ACE2 receptor, the mouse version of the protein does not bind to the SARS-CoV-2 spike. This means scientists would have a simple way to see if mouse cells were producing the human ACE2 protein, by seeing if the spikes bind to the cells.
Finding and expressing the ACE2 gene
To increase the chances that mouse cells would correctly incorporate and read the human ACE2 gene, the group used the intact gene. The genes of humans and other “higher organisms” contain a lot of information beyond the DNA sequence that codes for the building blocks of the amino acids that make up a protein. This additional information helps regulate the structure and function of genes within the cell’s chromosomes.
Scientists searched libraries of cloned DNA fragments generated as part of the Human Genome Project, a DOE-sponsored effort to map the location of all the genes that make us human, to find a fragment containing the ACE2 gene intact, complete. with its integrated regulatory information. Next, they exposed mouse cells to nanoparticles coated with this DNA fragment as well as the gene for another protein that makes the cells resistant to a deadly antibiotic.
“In this case, the nanoparticles serve as a DNA delivery agent that is engulfed by cells so that the DNA can potentially integrate into the chromosomes of mouse cells,” Freimuth said. “To find the cells that have taken up the foreign gene(s), we add the antibiotic to the cell cultures. The cells that have failed to take up and express the antibiotic resistance gene are died, while those that acquired resistance to antibiotics survived and grew into colonies.”.
The scientists grew about 50 of these colonies into individual cultures, then tested them to see how many also detected the human ACE2 gene and produced the human receptor protein.
Detection of protein production
“About 70% of the antibiotic-resistant colonies expressed the human ACE2 protein on the cell surface,” Freimuth said. “Further analysis showed that these colonies contained an average of 28 copies of the human ACE2 gene.”
Paul Freimuth (back), a virologist at Brookhaven Lab, led the effort to transform mouse cells to express human ACE2 receptor proteins. Feiyue Teng (front) of the lab’s Center for Functional Nanomaterials helped out in the biology lab and explored nanoscience-based applications for proteins. Credit: Roger Stoutenburgh/Brookhaven National Laboratory
Importantly, mouse cells retained the “foreign” copies of the ACE2 gene and continued to make the human ACE2 protein encoded by these genes for at least 90 cell generations.
The level of human ACE2 protein produced by the cells was generally proportional to the number of copies of the ACE2 gene integrated into the mouse genome. Several clones of mouse cells produced approximately 50 times more ACE2 than is normally present on mouse cells.
Scientists used various methods to test whether human ACE2 proteins made by mice were functional. This included demonstrating that a “pseudovirus” containing the COVID spike protein – that is, a non-pathogenic substitute for SARS-CoV-2 – could bind to receptors and infect cells.
“These infectivity assays showed that the human ACE2 protein expressed on these mouse cells is fully functional,” Freimuth said.
Uses and implications
Meanwhile, Oleg Gang and Feiyue Teng, co-authors of the CFN study, explored various ways to create extracellular nano-vesicles enriched with decoy human ACE2 for the potential treatment of COVID-19. They are also studying the placement of ACE2 proteins on nanoparticles for potential applications in treating infections or rapid detection of viruses.
“The challenge posed by ACE2-based nanovesicles lies in improving their neutralizing effect against SARS-CoV-2. We are also looking for ways to improve and exploit the binding sensitivity and specificity of ACE2-coupled nanoparticles to make them useful for the virus. Both approaches would require future optimization efforts,” said Teng, a research associate at CFN who has worked extensively on both the biological aspects of this study and potential nanoscience-based applications.
“We are excited to combine advances in nanomaterial fabrication with biomolecular approaches to develop new therapeutic and sensing strategies,” said Gang, who holds a joint appointment at Columbia University. “This study allowed us to overcome some methodological issues, because nanomaterials and biosystems required very different characterization approaches. What we learned here is important for our next steps to improve nanoparticle-based biosensing.”
In addition to enabling possible applications of the recombinant ACE2 protein, the work also demonstrates a new approach for producing a wide range of complex proteins. Examples include the vast array of cell surface receptors that mediate countless biological and pathological processes, as well as industrially important proteins such as monoclonal antibodies and enzymes.
“Our method of using intact genes along with mouse cells that can be adapted to grow in huge suspension cultures, much like liquid broth cultures used to grow bacteria, could advance large-scale production of these and other important proteins,” Freimuth said. .
More information:
Feiyue Teng et al, Overexpression of human ACE2 protein in mouse fibroblasts stably transfected with the intact ACE2 gene, Virology (2024). DOI: 10.1016/j.virol.2024.109988
Provided by Brookhaven National Laboratory
Quote: Scientists make COVID receptor protein in mouse cells (January 22, 2024) retrieved January 22, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.