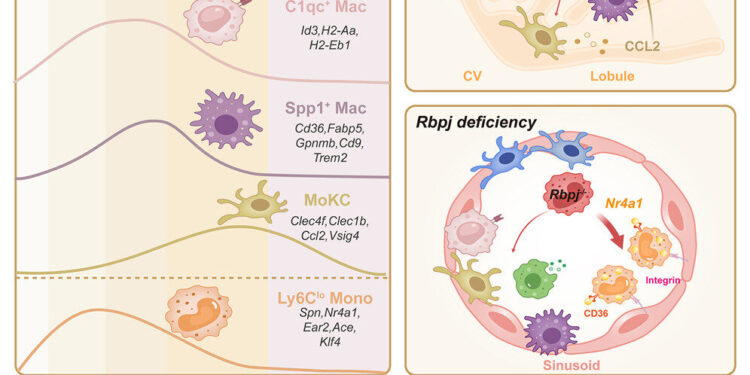
Graphical summary. Credit: Immunity (2024). DOI: 10.1016/j.immuni.2024.08.016
Scientists from the Agency for Science, Technology and Research (A*STAR) and the Shanghai Institute of Immunology (SII), along with their international collaborators, have dissected the key signaling mechanism behind origin of inflammation in metabolic dysfunction-associated steatohepatitis (MASH), thanks to which they also discovered a promising new protein target to combat fatty liver disease.
With a prevalence of 5.9% in Singapore and expected to rise to 7.5% in 2030, MASH is a chronic, progressive type of fatty liver disease in which the accumulation of fat in the liver triggers cell accumulation and response. immune system, causing inflammation. Furthermore, implicated by other increasing metabolic disorders (such as obesity and type 2 diabetes) as risk factors, it constitutes one of the main causes of liver-related morbidity and mortality, without any approved treatment is currently available to stop its progression.
In their article published in ImmunityDr Florent Ginhoux, Joint Principal Investigator at the A*STAR Singapore Immunology Network (A*STAR SIgN), with his team there and at SII, and their collaborators, used advanced single-cell RNA sequencing, tracing lineage and mouse models to identify that the Notch signaling pathway controls the conversion of monocytes into inflammatory macrophages, a process essential for the development of MASH.
By taking control of this pathway through the recombination signal binding protein for immunoglobulin Kappa J region protein (Rbpj), scientists were able to redirect the production of these inflammatory macrophages toward protective monocytes.
Shifting gears from inflammation to protection
The liver has its own native population of immune cells that adhere to endothelial cells surrounding hepatic capillaries. Together, they act as the guardians of immunity, filtering or removing harmful microorganisms, dead debris and lipids (fats) from the incoming blood.
However, when there are too many lipids, the environment becomes toxic, killing native immune cells and stressing endothelial cells to trigger an inflammatory response. Scientists have discovered how this calls non-native monocytes to the liver and how Notch signaling transforms them into inflammatory macrophages.
Digging deeper, the scientists also discovered that removing Rbpj, a seminal protein of the Notch pathway, not only prevents these non-native monocytes from transforming into inflammatory macrophages, but also allows them to transform into protective monocytes. These protective monocytes have more lipid-scavenging receptors and can absorb more lipids, helping to protect endothelial cells and calm inflammation.
A unique therapeutic target with dual function
This distinctive dual function of Rbpj makes it a key therapeutic target in the invention of new reparative strategies for MASH. The application of a simple treatment of nanoparticles carrying Rbpj inhibitors successfully delayed inflammation and lowered the grade of MASH, proving to be an extremely effective and targeted solution, without side effects.
“The Notch signaling pathway and the immune cells involved are not only well conserved between species, but also between organs and inflammatory diseases,” said Dr. Ginhoux. “With this knowledge, we aim to apply this study and treatment to other tissues and diseases with inflammatory pathways as the next stage of our research.”
This highly conserved nature of the Notch signaling pathway also presents optimistic prospects for clinical testing, as there is a good chance that successful treatments in mice could be effectively translated to human patients.
Current treatments are primarily focused on metabolic mediation, but by targeting Rbpj in the Notch signaling pathway, it is hoped that a new class of therapies directly addressing the underlying factors of MASH could be made available.
More information:
Wei Guo et al, Notch signaling regulates macrophage-mediated inflammation in steatotic liver disease associated with metabolic dysfunction, Immunity (2024). DOI: 10.1016/j.immuni.2024.08.016
Provided by Agency for Science, Technology and Research (A*STAR), Singapore
Quote: Scientists identify a key protein in the inflammatory pathway causing fatty liver disease (October 15, 2024) retrieved October 15, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.