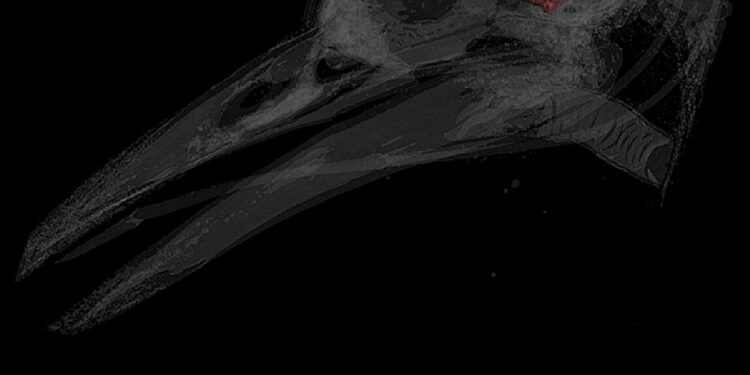
Digital reconstructions of endocasts of a peak, Melanerpes aurifrons (top) and a troodontid dinosaur, Zanabazar junior (down). The blue area is the cerebellum. Credit: Amy Balanoff
Evolutionary biologists at Johns Hopkins Medicine report that they have combined PET scans of modern pigeons with studies of dinosaur fossils to help answer a lingering question in biology: How did bird brains evolve to allow them to fly ?
The answer, they say, appears to be an adaptive increase in cerebellum size in some fossil vertebrates. The cerebellum is a region of the brain responsible for movement and motor control.
The research results are published in the January 31 issue of Proceedings of the Royal Society B.
Scientists have long thought that the cerebellum should play an important role in bird flight, but they lacked direct evidence. To identify its value, the new research combined modern PET imaging data from ordinary pigeons with fossil records, examining the brain regions of birds during flight and the brain boxes of ancient dinosaurs.
“Powered flight among vertebrates is a rare event in evolutionary history,” says Amy Balanoff, Ph.D., assistant professor of functional anatomy and evolution at Johns University School of Medicine. Hopkins and first author of the published research.
In fact, Balanoff says, only three groups of vertebrates, or animals with backbones, evolved to fly: the extinct pterosaurs (terrors of the sky during the Mesozoic period, which ended more than 65 years ago millions of years), bats and birds.
The three species are not closely related on the evolutionary tree, and the key drivers or factors that enabled flight in all three remain unclear.
In addition to external physical adaptations for flight, such as long upper limbs, certain types of feathers, a streamlined body, and other features, Balanoff and his colleagues designed research to find features that created a flight-ready brain.
To do this, she worked with biomedical engineers at Stony Brook University in New York to compare the brain activity of modern pigeons before and after flight.
The researchers performed positron emission tomography, or PET, the same technology commonly used in humans, to compare activity in 26 brain regions when the bird was at rest and immediately after flying for 10 minutes of one perch to another. They scanned eight birds on different days.
PET scans use a compound similar to glucose that can be tracked where it is most absorbed by brain cells, indicating increased use of energy and therefore activity. The tracker breaks down and is excreted from the body within a day or two.
Of the 26 regions, one area – the cerebellum – showed a statistically significant increase in activity levels between rest and flight in all eight birds. Overall, the level of increased activity in the cerebellum differed by more than two standard statistical deviations from other areas of the brain.
The researchers also detected increased brain activity in optic flow pathways, a network of brain cells that connect the retina of the eye to the cerebellum. These pathways process movement across the visual field.
Balanoff says their findings of increased activity in the cerebellum and optic flow pathways were not necessarily surprising, since these areas are thought to play a role in flight. What was new in their research was linking findings about the cerebellum of the flight-capable brains of modern birds to the fossil record that showed how the brains of bird-like dinosaurs began to develop brain conditions for powered flight.
To do this, Balanoff used a digitized database of endocasts, or casts of the internal space of dinosaur skulls, which, when filled, resemble the brain. She identified and traced a dramatic increase in cerebellum volume in some of the earliest species of maniraptoran dinosaurs, which preceded the first appearances of powered flight in ancient bird relatives, including Archeopteryx, a winged dinosaur.
Balanoff and his team also found evidence in the endocasts of increased tissue folding in the cerebellum of early maniraptorans, an indication of increasing brain complexity.
The researchers cautioned that these were early findings and that changes in brain activity during powered flight could also occur during other behaviors, such as gliding. They also note that their tests involved simple flight, without obstacles and with an easy flight path, and that other regions of the brain might be more active during complex flight maneuvers.
The research team next plans to identify specific areas of the cerebellum that enable a flight-ready brain as well as the neural connections between these structures.
Scientific theories for why the brain grew larger throughout evolutionary history include the need to traverse new and different landscapes, paving the way for flight and other styles of locomotive, says Gabriel Bever, Ph. D., associate professor of functional anatomy and evolution at Johns University. Hopkins University School of Medicine.
“At Johns Hopkins, the biomedical community has a wide range of tools and technologies to help us understand evolutionary history and connect our discoveries to basic research on how the brain works,” adds- he.
More information:
Quantitative functional imaging of the pigeon brain: implications for the evolution of avian powered flight, Proceedings of the Royal Society B: Biological Sciences (2024). DOI: 10.1098/rspb.2023.2172. royalsocietypublishing.org/doi….1098/rspb.2023.2172
Provided by Johns Hopkins University School of Medicine
Quote: Scientists identify growth of the brain’s cerebellum as key to the evolution of bird flight (January 30, 2024) retrieved January 31, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.