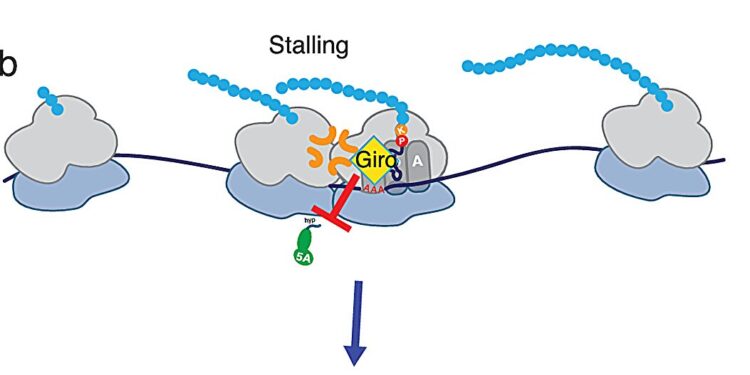
Model of translational modulation mediated by Giro and eIF5A. Credit: Natural communications (2025). DOI: 10.1038/s41467-024-54838-2
Girollina, a compound extracted from the sea sponge Pseudaxinyssa cantharella, has been studied for possible antitumor effects and has also been found to have antimalarial effects.
Now, thanks to the work of scientists at the RIKEN Center for Sustainable Resource Science, researchers have a better idea of how it works. In addition to its possible medicinal properties, the current findings suggest that the compound could also be useful as a chemical probe for research in areas such as aging and mitochondrial health.
Girollina is one of several compounds with biological functions isolated from Pseudaxinyssa cantharella. Sea sponges are important sources of biological compounds because they are unable to move autonomously and must protect themselves by chemical means.
The possible anti-tumor effect of girollin was initially thought to be because it was a general translation inhibitor, meaning that it prevented the translation of messenger RNA into the amino acid sequence of proteins. by ribosomes, the cell’s protein production factories. However, its effects and the mechanisms underlying them have subsequently been little explored.
In the current work, published in Natural communicationsScientists have used new techniques to better understand how girollina works.
According to Tilman Schneider-Poetsch of the RIKEN Center for Sustainable Resource Science, the corresponding author of the article, “Through our work, we were able to discover that girollina acts through a new mechanism of action. This is the first small molecule that has been found to directly modulate the function of an important protein synthesis factor and to act selectively on certain amino acid sequences, rather than as a general inhibitor of protein translation. ‘RNA in proteins.
Essentially, what they found is that girollin works by modulating the activity of eIF5A, a translation elongation factor that helps the ribosome navigate difficult-to-translate amino acid sequences. When ribosome speed slows, eIF5A binds and increases the rate of amino acid incorporation, preventing ribosomes from stalling.
Girollina prevents eIF5A from binding to the ribosome. Without the help of eIF5A, the ribosome will get stuck on certain sequences. Stalled ribosomes are then attacked by the ribosome-associated quality control (RQC) pathway, which degrades the protein being produced. This premature degradation of unfinished proteins seems to explain, to some extent, the toxicity of girollina.
Sequences that cause the blockage include those encoding the amino acids proline and lysine, particularly when lysine is encoded by the RNA sequence AAA (three consecutive adenine bases).
This finding is expected to have implications for the compound’s effect on malaria, because the messenger RNA molecules of Plasmodium falciparum, the parasite that causes malaria, tend to have many adenine bases, making them susceptible to the effects of the girollina.
As eIF5A is known to play a role in maintaining mitochondrial function and its dysfunction has been implicated in the aging process, girollina should also provide a useful chemical tool for dissecting the function of eIF5A in maintenance and aging. mitochondria.
According to Schneider-Poetsch, “Girolline, due to its selectivity, is a new tool that we can use to study the role of eIF5A, an important protein involved in aging, neurodegeneration and cancer. plans to investigate this topic.
More information:
Tilman Schneider-Poetsch et al, Girolline is a selective sequence context modulator of eIF5A activity, Natural communications (2025). DOI: 10.1038/s41467-024-54838-2
Quote: Scientists explain how a compound in a sea sponge exerts its biological effects (January 14, 2025) retrieved January 14, 2025 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.