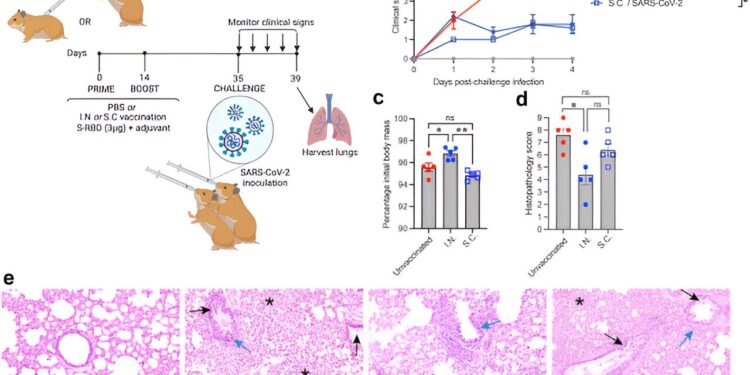
Mucosal vaccination protects hamsters from SARS-CoV-2-induced clinical disease. (a) Schematic of the experimental design in which hamsters were vaccinated with S-RBD and M7 via various routes using priming (day 0) and boosting (day 14). Credit: eBioMedicine (2023). DOI: 10.1016/j.ebiom.2023.104924
A team of scientists, led by Duke-NUS Medical School, has discovered a potential intranasal vaccine candidate that confers improved and longer-lasting immunity against SARS-CoV-2 viruses compared to administration by injection. By triggering an immune response directly at the point of entry, the intranasal vaccine candidate improved the long-term immune memory of the virus, which could translate into a reduction in the need for booster shots.
There is growing evidence that intranasal vaccines provide better protection at mucosal surfaces, making this a vaccination route that could reduce breakthrough infections and subsequent transmission of the virus.
To explore this topic further, the research team, which includes collaborators from Duke-NUS’s parent universities – Duke University and the National University of Singapore – among others, compared the immune responses resulting from nasal and sub-nasal administration. skin of the vaccine, as well as the immunity of the vaccine with and without the use of adjuvants, that is, substances added to vaccines to strengthen the body’s immune response.
Published in eBioMedicine, results showed that nasal administration of the vaccine candidate stimulated the mucosal antibody response, as expected. Additionally, and more importantly, it enhanced longer-term mucosal and systemic immune protection through the preferential induction of airway-resident T cells and central memory T cells.
“Our data shows that, compared to subcutaneous vaccination, the intranasal route improved the response of certain immune cells, called T cells, which reduced the severity of the disease,” explained Associate Professor Ashley St John , from the Duke-NUS Emerging Infectious Diseases Program. who is the lead author of the study.
“Not only that, but it also resulted in greater numbers of central memory T cells compared to subcutaneous vaccination, which could lead to longer-lasting protection.”
Central memory T cells play an essential role in protecting the body in the event of re-exposure to a virus. They improve the memory of the immune system, inducing long-lasting protective immune responses. This ability to retain this long-term memory of the virus suggests that there is less need for pathogenic challenge to achieve the same level of protection against the virus, which could result in fewer boosters.
The research team also discovered that the use of adjuvants in the vaccine to promote the immune response influenced the characteristics of T cells, as well as their activation and production of cytokines (tiny proteins that regulate cellular communication and control inflammation), different adjuvants leading to different effects. T cell responses.
Another notable finding of the study is that a type of antibody, called IgG, that circulates widely in the bloodstream is more effective at neutralizing virus variants, including newly emerging ones, when induced by nasal vaccination route. These findings provide important scientific evidence that enhanced T cell and IgG antibody responses contribute to greater and long-lasting protection from intranasal COVID-19 vaccines.
“Although the acute phase of the pandemic may be behind us, the rise of new variants, including JN.1, which has triggered an increase in hospitalizations locally, demonstrates that we have room in our vaccine arsenal and treatments for even better tools. This study shows that mucosal vaccination holds promise for improving COVID-19 vaccine effectiveness with potentially fewer boosters needed,” said Professor Patrick Tan, Senior Associate Dean for Research at Duke-NUS.
A patent has been filed on the discovery, which covers the invention of the vaccine composition formulated for mucosal delivery, paving the way for an industrial partnership to potentially develop mucosal vaccines against COVID-19 and other pathogens which also target mucosal surfaces.
More information:
O’Neill A, Mantri CK, Tan CW, Saron WA, Nagaraj SK, Kala MP, Joy CM, Rathore APS, Tripathi S, Wang LF, St. John AL. Mucosal SARS-CoV-2 vaccination of rodents elicits higher systemic central memory T function and cross-neutralizing antibodies against variants of concern. eBioMedicine (2024). DOI: 10.1016/j.ebiom.2023.104924.
Provided by Duke-NUS Medical School
Quote: Scientists discover potential nasal COVID-19 vaccine candidate that provides better, longer protection (January 9, 2024) retrieved January 9, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.