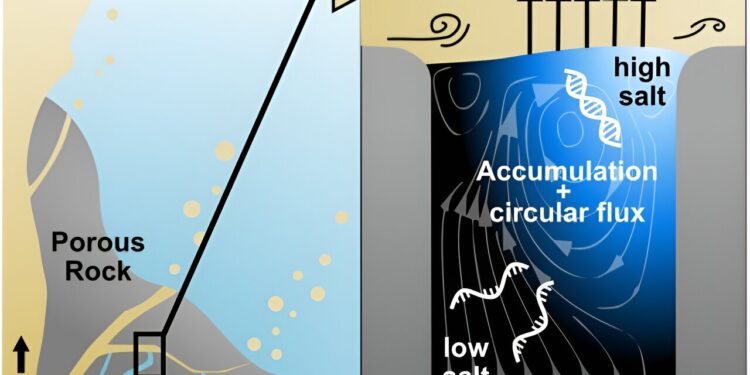
Replication at the gas-water interface. We considered a geological scenario in which water, containing biomolecules, is evaporated by a millimeter-scale gas flow. In porous volcanic rocks, many such settings can be imagined. The gas flow induces convective water currents and causes its evaporation. Dissolved nucleic acids and salts accumulate at the gas-water interface due to interfacial currents, even if the inflow from below is pure water. Through the induced vortex, nucleic acids pass through different salt concentrations, promoting separation of the strands and allowing them to replicate exponentially. Our experiments reproduce this environment on a microscopic scale, by subjecting a defined sample volume to a continuous influx of pure water with a flow of air passing through it. Credit: eLife (2024). DOI: 10.7554/eLife.100152.1
Researchers have discovered a plausible evolutionary framework in which nucleic acids – the fundamental genetic building blocks of life – could enable their own replication, eventually leading to life on Earth.
The study, published today as a revised preprint in eLifewas described by the editors as an important work with compelling evidence showing how a simple geophysical setting of a gas flow over a narrow water channel can create a physical environment leading to nucleic acid replication. This work will be of interest to scientists working on the origin of life and, more broadly, on nucleic acids and diagnostic applications.
The emergence of life on Earth remains an unsolved enigma, but a common theory is that the replication of genetic material – the nucleic acids DNA and RNA – is a central and critical process. RNA molecules can store genetic information and catalyze their own replication by forming double-stranded helices. The combination of these abilities allows them to mutate, evolve and adapt to diverse environments and, ultimately, encode the building blocks of life’s proteins.
For this to happen, the RNA strands must not only replicate in a double-stranded form, but also separate again to complete the replication cycle. Strand separation, however, is a difficult task at the high salt and nucleic acid concentrations required for replication.
“Various mechanisms have been studied for their potential to separate the DNA strands at the origin of life, but they all require temperature changes that would lead to degradation of nucleic acids,” explains lead author Philipp Schwintek, a doctoral student. . student in systems biophysics at the Ludwig-Maximilians-Universität München, Munich, Germany.
“We studied a simple and ubiquitous geological scenario in which the movement of water through a rock pore was dried by a gas percolating through the rock to reach the surface. Such a setting would be very common on the volcanic islands of the early Earth which provided the dry conditions necessary for RNA synthesis.”
The team built a laboratory model of rock pores exhibiting an upward flow of water evaporating at an intersection with a perpendicular gas flow, which leads to a buildup of dissolved gas molecules at the surface. At the same time, the gas flow induces circular currents in the water, pushing molecules back into the mass. To understand how this model would affect nucleic acids in the environment, they used beads to monitor water flow dynamics and then tracked the movement of short fluorescently labeled DNA fragments.
“We expected that continued evaporation would lead to an accumulation of DNA strands at the interface,” says Schwintek. “Indeed, we found that water continually evaporated at the interface but that nucleic acids from the aqueous side accumulated near the gas/water interface.” Five minutes after the start of the experiment, the accumulation of DNA strands was three times greater, while after one hour there were 30 times more DNA strands accumulated at the interface.
Although this suggests that the gas/water interface allows a sufficient concentration of nucleic acids for replication to occur, separation of DNA double strands is also necessary. Usually a change in temperature is necessary, but when the temperature is constant, changes in salt concentration are necessary.
“We hypothesized that the circular fluid flow at the interface provided by the gas flow, alongside passive diffusion, would drive strand separation by forcing nucleic acids through areas with different salt concentrations” , explains lead author Dieter Braun, professor of systems biophysics at Ludwig. -Maximilians-University of Munich.
To test this, they used a method called FRET spectroscopy to measure the separation of DNA strands: a high FRET signal shows that the DNA strands are still linked, while a low FRET signal indicates that the strands are separated. . As expected, the FRET signal increased initially near the gas–water interface, indicating the formation of double-stranded DNA. But during the experiment, where there was an upward flow of water, the FRET signal was weak, indicating single-stranded DNA.
Additionally, when the team overlaid this data with their simulation of water flow and salt concentrations, they found that the vortex at the gas-water interface caused up to three times higher changes in the salt concentrations, potentially capable of causing strand separation.
Although nucleic acids and salts accumulated near the gas-water interface, in most of the water, salt and nucleic acid concentrations remained extremely low. This prompted the team to test whether nucleic acid replication could actually occur in this environment, by adding nucleic acids labeled with a fluorescent dye and an enzyme capable of synthesizing double-stranded DNA into the laboratory model of the pore of the rock. Unlike normal laboratory DNA synthesis reactions, the temperature was kept at a constant temperature and the reaction was exposed to the combined influx of water and gas.
After two hours, the fluorescent signal had increased, indicating an increased number of replicated double-stranded DNA molecules. Yet, when the influx of gas and water was cut off, no increase in fluorescence signals was observed, and therefore no increase in double-stranded DNA was observed.
“In this work, we investigated a plausible and abundant geological environment that could trigger the replication of early life stages,” concludes Braun. “We considered an environment of gas flowing over an open rock pore filled with water, without any temperature change, and found that the combined flow of gas and water can trigger salt fluctuations that promote the replication of DNA.
“As this is a very simple geometry, our findings greatly expand the repertoire of potential environments that could enable replication on early planets.”
More information:
Philipp Schwintek et al, Prebiotic gas flow environment enables isothermal nucleic acid replication, eLife (2024). DOI: 10.7554/eLife.100152.1
Journal information:
eLife
Quote: Scientists discover plausible geological context that could have sparked life on Earth (October 1, 2024) retrieved October 2, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.