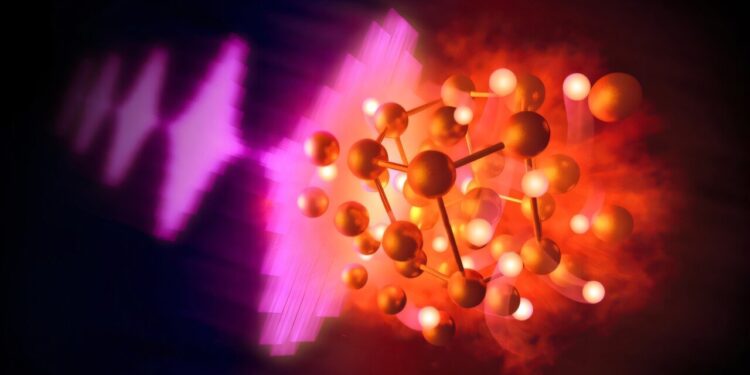
Illustration of the intense pulses of an X -ray X -ray laser (on the left) heating samples compressed of hydrocarbons under extreme conditions, resulting in the reaction of gold and hydrogen to form gold hydride (center). The gold atoms, represented in gold, are fixed in a hexagonal crystalline network through which hydrogen, shown in white, freely diffuses in a “superiony” state. Credit: Greg Stewart / SLAC National Accelerator Laboratory
In Serendian way and for the first time, an international research team led by scientists from the National Laboratory of National Accelerator of the American Department of Energy formed a solid binary gold hydride, a compound exclusively made of gold and hydrogen atoms.
The researchers studied how long it takes hydrocarbons, carbon and hydrogen compounds, to form diamonds under extremely high pressure and heat.
In their experiences in the European XFEL (laser of Franc-Rayon electrons) in Germany, the team studied the effect of these extreme conditions in hydrocarbon samples with an integrated gold leaf, which was intended to absorb X-rays and to heat the hydrocarbons weakly absorbent. To their surprise, they not only saw the formation of diamonds, but also discovered the formation of gold hydride.
“It was unexpected because gold is generally chemically very boring and non -reactive – that is why we use it as an X -ray absorber in these experiences,” said Mungo Frost, scientist of SLAC staff who led the study.
“These results suggest that there are potentially a lot of new chemistry to discover in extreme conditions where the effects of temperature and pressure are starting to compete with conventional chemistry, and you can train these exotic compounds.”
The results, published in International edition of Angewandte ChemieGive an overview of how chemistry rules change in extreme conditions such as those found inside certain hydrogen fusion planets or stars.
Study dense hydrogen
In their experience, the researchers first pressed their hydrocarbon samples to pressures greater than those of the Earth coat using a diamond anvil cell. Then, they heated the samples to more than 3,500 degrees Fahrenheit by hitting them several times with European XFEL XFEL XFEL pulses.
The team recorded and analyzed how the radiographs dispersed the samples, which allowed them to resolve the structural transformations inside.
As expected, recorded broadcasting models have shown that carbon atoms had formed a diamond structure. But the team also saw unexpected signals which were due to hydrogen atoms reacting with the gold leaf to form gold hydride.
Under the extreme conditions created in the study, the researchers noted that hydrogen was in a dense “superon” state, where hydrogen atoms flowed freely through the rigid atomic network of gold, increasing the conductivity of gold hydride.
Hydrogen, which is the lightest element of the periodic table, is difficult to study with X -rays because it only diffuses X -rays weakly. Here, however, superonic hydrogen interacted with much heavier gold atoms, and the team was able to observe the impact of hydrogen on the way in which the gold network dispersed the X -rays.
“We can use the gold network as a witness for what hydrogen does,” said Mungo.
The gold hydride offers a means of studying dense atomic hydrogen under conditions which could also apply to other situations which are not directly accessible directly. For example, dense hydrogen is the interiors of certain planets, so studying it in the laboratory could teach us more about these foreign worlds.
It could also provide new perspectives on nuclear fusion processes inside stars like our sun and help develop technology to exploit fusion energy here on earth.
Explore a new chemistry
In addition to paving the way for dense hydrogen studies, research also offers a way to explore a new chemistry. Gold, which is generally considered as a non -reactive metal, has proven to form a stable hydride at an extremely high pressure and temperature.
In fact, it only seems to be stable under these extreme conditions, as when it cools, gold and hydrogen separate. Simulations have also shown that more hydrogen can adapt in the golden network to higher pressure.
The simulation frame could also be extended beyond gold hydride.
“It is important that we can experimentally produce and model these states under these extreme conditions,” said Siegfried Glenzer, director of the High Energy Density Division and Slac Photons Professor and Principal Researcher of the Study.
“These simulation tools could be applied to model other properties of exotic materials under extreme conditions.”
More information:
Mungo Frost et al, synthesis of high pressure gold hydride and high temperature, International edition of Angewandte Chemie (2025). DOI: 10.1002 / Anie.202505811
Supplied by SLAC National Accelerator Laboratory
Quote: Scientists create gold hydride by combining gold and hydrogen in extreme conditions (2025, August 5) recovered on August 5, 2025 from
This document is subject to copyright. In addition to any fair program for private or research purposes, no part can be reproduced without written authorization. The content is provided only for information purposes.