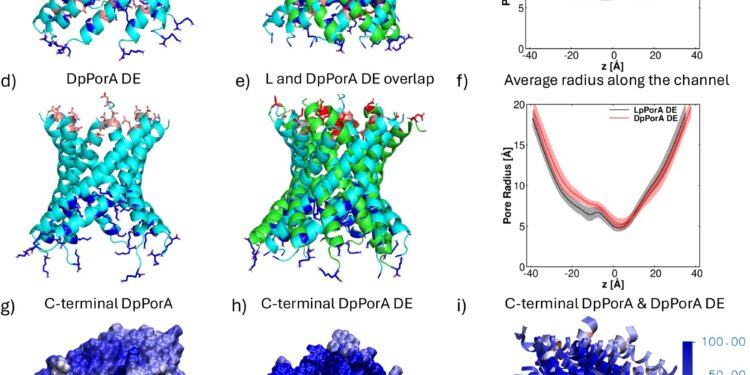
Structures and radius profiles along the channel axis of the designed pores. Credit: Natural communications (2025). DOI: 10.1038/s41467-025-64025-6
For the first time, researchers have successfully fabricated and characterized a fully functional mirror-image nanopore, a molecular gateway constructed entirely from D-amino acids, the mirror-image forms of the natural building blocks of proteins. The work, led by Professor Kozhinjampara R. Mahendran of the Rajiv Gandhi Center for Biotechnology (India) in collaboration with Constructor University and other partners, not only demonstrates a major milestone in nanoscience, but also paves the way for promising biomedical applications, including potential cancer therapies.
In nature, proteins are almost exclusively built from L-amino acids, while their D-amino acid counterparts generally play only minor roles. Building entire proteins from D-amino acids is extremely difficult, but offers striking advantages: such mirror-image structures are often more resistant to degradation and can interact differently with biological systems.
In this study, the team designed a stable and well-defined synthetic peptide D pore called DpPorA. Remarkably, by changing the charge distribution, they were able to create higher versions of these pores with improved conductance and selectivity under different salt conditions.
Experiments have revealed that these pores can detect a broad spectrum of biomolecules at the single-molecule level, including peptides, cyclic sugars, and certain proteins, including one that is central to Parkinson’s disease research. Fluorescence imaging confirmed that pores form large, flexible channels in membranes, allowing size-dependent transport of molecules.
“Our simulations provided the molecular-level picture needed to prove that these mirror-image pores are the exact counterparts of their natural analogues,” says Professor Ulrich Kleinekathöfer, professor of physics at Constructor University Bremen and co-author of the study published in Natural communications. “This understanding was key to explaining the experiments and will guide the design of improved pore variants in the future.”
Simulations performed by Constructor University scientists were key to verifying the mirror-image architecture of the pore. Comparing the D pore with its natural L counterpart, molecular dynamics studies confirmed that both are perfect structural reflections, while also explaining the subtle differences in conductance and selectivity observed in the experiments.
“The computational work gave us confidence that we were indeed looking at a real mirror-image pore,” says Dr. Kalyanashis Jana, a postdoctoral researcher in Kleinekathöfer’s group and also first author of the paper.
Beyond basic science, the results suggest exciting biomedical potential. In cellular studies, fluorescently labeled mirror-image pores showed potent membrane-disrupting effects in cancer cells but had no impact on normal cells, portending selective cytotoxicity that could one day be exploited for cancer treatment.
The study also reflects the continuity of scientific collaboration across borders and generations. Mahendran and his colleague Dr. Harsha Bajaj received their doctorates from Professor Mathias Winterhalter at the former Jacobs University in Bremen (now Constructor University). Today, they continue to collaborate with Constructor University scientists, illustrating the enduring networks of expertise that drive scientific progress.
More information:
Neilah Firzan CA et al, Fabrication of mirror-image cytotoxic nanopores, Natural communications (2025). DOI: 10.1038/s41467-025-64025-6
Powered by Constructor University
Quote: Revolutionary mirror-image nanopores open the door to new biomedical applications (October 11, 2025) retrieved October 13, 2025 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.