MXene nanoparticles (MXene NPs) promote calcium deposition around cells, which triggers the upregulation of iNOS and SGK1 which contribute to muscle growth. Credit: Associate Professor Yun Hak Kim / Pusan National University.
Tissue engineering, which involves the use of grafts or scaffolds to facilitate cellular regeneration, is emerging as a key medical practice for treating volumetric muscle wasting (VML), a condition in which a significant amount of muscle tissue is lost beyond the body’s natural regenerative capacity. To improve surgical outcomes, traditional muscle grafts are giving way to artificial scaffolding materials, with MXene nanoparticles (NPs) emerging as a promising option.
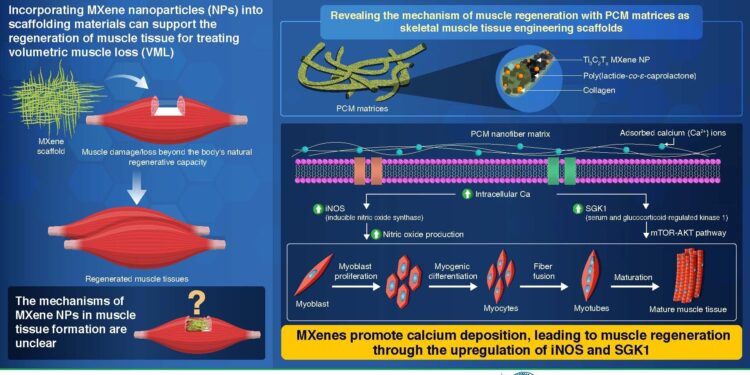
MXene NPs are 2D materials primarily composed of transition metal carbides and nitrides. They are highly electrically conductive, can accommodate a wide range of functional groups, and have stacked structures that promote cellular interactions and muscle growth. Although practical laboratory demonstrations have shown their ability to promote skeletal muscle rebuilding, the specific mechanism by which they achieve this remains unclear.
To fill this gap, Associate Professor Yun Hak Kim from the Department of Anatomy and the Department of Biomedical Informatics, alongside Professors Suck Won Hong and Dong-Wook Han from the Department of Cognitive-Mechatronics Engineering at the National University of Pusan, have developed nanofibrous matrices containing MXene NPs. as scaffolding. They used DNA sequencing to reveal the genes and biological pathways activated by MXene NPs to facilitate muscle regeneration.
These results, published in Nano-Micro Lettersmark a significant advance in the use of MXene scaffolds to treat muscle injuries.
“This discovery opens a prospective avenue for the use of these materials to increase the efficiency of muscle tissue regeneration after injury or damage,” explains Professor Kim.
In the initial phase, the team created a nanofibrous PCM matrix containing poly(lactide-co-ε-caprolactone) (P), reinforced with collagen (C), and Ti3VS2TX MXene (M) nanoparticles. To determine the specific effect of MXene NPs on muscle growth, they prepared three controls: blank PLCL (P), PLCL with collagen (PC), and PLCL with MXene (PM). By testing all scaffolds in mouse models with induced volumetric muscle loss, the researchers observed a significant increase in overall muscle cell numbers in PCM-treated mice compared to other groups.
To understand the impact of MXene nanoparticles (NPs) on muscle regeneration and growth at the molecular level, researchers introduced C2VS12 myoblasts, precursors of muscle cells, on PC and PCM matrices. The objective was to analyze the differences in gene expression levels between the two matrices. Within the PCM matrix, increased production of inducible nitric oxide synthase (iNOS) and serum/glucocorticoid-regulated kinase 1 (SGK1) was identified, two proteins closely associated with calcium signaling and muscle regeneration.
These results suggest that MXenes favor the calcium ion (Ca2+) deposit around the cells. This increases intracellular Ca levels2+ triggers the activation of genes that produce iNOS and SGK1 proteins. SGK1 influences the mTOR-AKT pathway, promoting cell proliferation, survival and myogenesis, the conversion of myoblasts into muscle fibers. Simultaneously, iNOS increases nitric oxide (NO) production, thereby contributing to myoblast proliferation and muscle fiber fusion.
The combined effects lead to the development of mature muscle tissue. Aligned PCM nanofibrous matrices offer biophysical cues for intracellular biochemical signaling, guiding myogenic behaviors. This finding contributes to our understanding of MXene’s potential to regrow muscles and holds promise for refining scaffold design to further improve this process.
“Within 5 to 10 years, this research could lead to breakthrough treatments for muscle injuries. MXene NP-infused matrices could become routine in medical practice for athletes, people with muscle diseases, and those who are recovering from trauma or muscle surgery,” says Professor Kim. “These NPs could improve methods of muscle regeneration, providing better outcomes for reconstructive surgeries and conditions such as muscular dystrophy, where muscle function is compromised.”
MXene NP-infused matrices offer potential for customization to meet various needs in the treatment of muscle wasting injuries. This customization may involve adjusting the composition, structure, or properties to meet specific patient requirements, such as size, shape, or enhancement of bioactivity. Adaptation of these materials could offer personalized solutions to different levels of muscle loss. Additionally, the observed enhanced muscle regeneration could contribute to more efficient recovery, potentially reducing the need for post-treatment rehabilitation.
These matrices, with controllable mechanical properties, are promising for improving muscle regeneration in vivo. Further research on MXene promises expanded clinical applications, potentially beneficial to human well-being.
More information:
Moon Sung Kang et al, Highly aligned ternary nanofiber matrices loaded with MXene accelerate regeneration of volumetric muscle loss, Nano-Micro Letters (2024). DOI: 10.1007/s40820-023-01293-1
Provided by Pusan National University
Quote: Researchers discover the molecular mechanisms behind the effects of MXene nanoparticles on muscle regeneration (January 24, 2024) retrieved January 24, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.