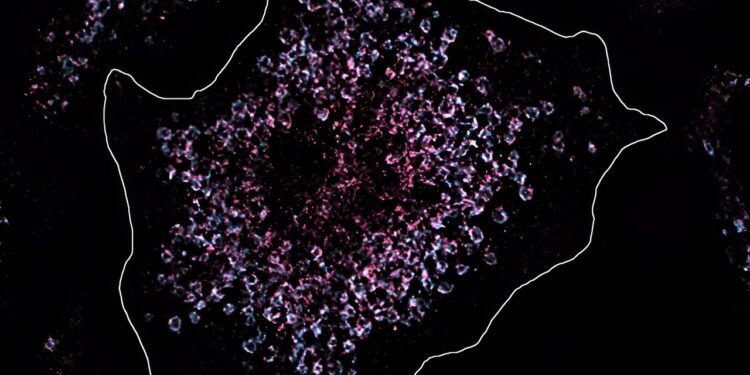
In this cell, Toll-like receptor 7 was colored pink in order to analyze its quantity and position under the microscope. The cell is healthy and the quantity of Toll-like receptor is normal. The situation is different in the immune cells of lupus patients, which have a significantly increased number of receptors, through which the body’s genetic material is recognized and chronic lupus inflammation is triggered. Credit: MPI f. Biology of infections/Fenja Blank
Sometimes just one mutation in our genetic makeup is enough to cause disease. This is also the case for lupus, an autoimmune disease. Lupus causes severe inflammation throughout the body and can have serious consequences on the lives of those affected. Researchers at the Max Planck Institute for Infection Biology in Berlin have discovered a mechanism that may trigger lupus in children.
This mechanism regulates the amount of a specific immune receptor that normally recognizes the genetic material of pathogens. If this mechanism is disrupted, receptors accumulate in immune cells leading to the recognition of the organism’s genetic material. As a result, the immune system turns against its own body and causes the systemic inflammation of lupus. In collaboration with doctors at the Ludwig Maximilian University Hospital in Munich, the researchers were able to identify a lupus patient in whom this mechanism is disrupted by a single mutation.
The innate immune system responds to invading pathogens within minutes. This is a first line of defense that keeps pathogens at bay until the more specific adaptive immune system kicks in. However, this speed comes at a price: the innate immune system response is so strong and not very specific that it must be well controlled to prevent it. to turn against the body itself.
In Olivia Majer’s group at the Max Planck Institute for Infection Biology, researchers are working to better understand these control mechanisms of the innate immune system. The group is focusing on an immune receptor called Toll-like receptor 7, which can recognize the genetic material of viruses and bacteria and then trigger an immune response against invaders.
Imbalanced immune receptor
For the immune system to respond quickly, a certain number of these receptors must be present in immune cells. Cells maintain this balance by constantly producing and degrading receptors. “We wanted to understand what happens when this balance is disrupted,” explains group leader Olivia Majer.
During their work, Majers’ team became interested in a protein complex called BORC. The researchers were able to show that BORC is necessary to degrade Toll-like receptor 7 within the cell. Additionally, BORC requires another protein, UNC93B1, to carry out the degradation process. If there is an error in this process, the receptor is not degraded and accumulates in immune cells.
“From previous experiments on mice done a few years ago at the University of Berkeley in California, we already knew that too many of these receptors were a problem,” says Majer. A greater number of receptors bias the recognition of the body’s own genetic material. This leads to an immune response against oneself, triggering the autoimmune disease lupus. However, so far neither BORC nor UNC93B1 have been associated with lupus in humans.
The researchers received confirmation of their findings by telephone. Fabian Hauck teaches, researches and treats patients at the Ludwig Maximilian University Hospital in Munich and specializes in congenital immune disorders such as lupus. He became aware of Majer’s research because one of his patients had a mutation in the gene for a previously unnoticed protein: UNC93B1.
It is precisely this protein that Majer had identified with his team. “When I received the first call from Fabian Hauck, I thought it was too good to be true,” explains Majer, “but in eight weeks of joint efforts, we were able to confirm that the UNC93B1 mutation The cause of this problem was the patient’s lupus.
A new approach to lupus therapies
Hauck and Majer’s findings were published in the journal Scientific immunology. At the same time, the journal also publishes the work of a research group from the Technical University of Dresden, with which the two researchers collaborate. In this study, Dresden scientists identified additional mutations in UNC93B1 that may trigger lupus. Researchers have discovered a completely new mechanism that triggers a particularly aggressive form of lupus: severe symptoms develop in early childhood, while many other forms of lupus only appear in adults.
The search for mutations in UNC93B1 could quickly become part of the treatment of lupus, opening the way to new therapeutic approaches. In the past, doctors mainly focused on suppressing inflammation using medications. By targeting the newly discovered mechanism, it may be possible to prevent inflammation from developing and thus significantly reduce the disease burden for those affected.
More information:
Harshita Mishra et al, Disrupted TLR7 degradative sorting is associated with human lupus, Scientific immunology (2024). DOI: 10.1126/sciimmunol.adi9575
Christine Wolf et al, UNC93B1 variants cause TLR7-dependent autoimmunity, Scientific immunology (2024). DOI: 10.1126/sciimmunol.adi9769
Provided by the Max Planck Society
Quote: Researchers trace a form of lupus to a single mutation (January 12, 2024) retrieved on January 12, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.