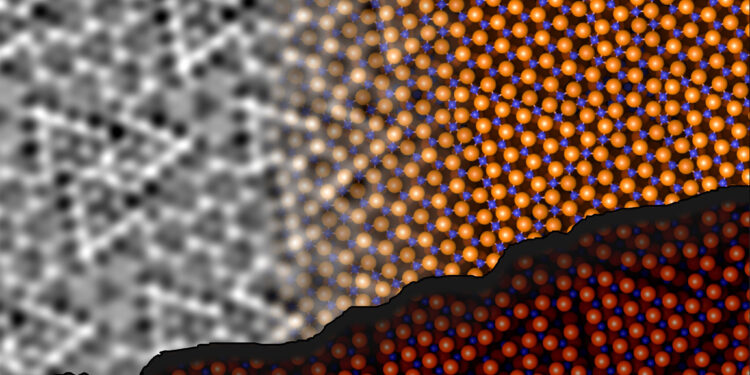
The structure of the aluminum oxide surface was determined by non-contact atomic force microscopy and computer modeling. Credits: Johanna Hütner, David Kugler, Jan Balajka
Aluminum oxide (Al2O3), also known as alumina, corundum, sapphire or ruby, is one of the best insulators used in a wide range of applications: in electronic components, as a support material for catalysts or as a chemically resistant ceramic, to name a few.
Knowing the precise arrangement of atoms on the surface is essential to understanding how chemical reactions take place in this material, such as those in catalytic processes. The atoms inside the material follow a fixed arrangement, giving rise to the characteristic shapes of the crystals.
On the surface, however, the structure differs from that of the crystal interior. The highly insulating nature of alumina has hampered experimental studies and the surface structure has eluded precise determination for more than half a century. Researchers at TU Wien and the University of Vienna have now solved the complex structure of aluminium2O3 surface, a puzzle listed in 1997 as one of the “three mysteries of surface science.”
The research group led by Jan Balajka and Ulrike Diebold published its findings in Science.
High-resolution microscopy identifies surface atoms
The research team used non-contact atomic force microscopy (ncAFM) to analyze the surface structure. This method generates images of the surface structure by scanning a sharp tip mounted on a quartz tuning fork a short distance from the surface. The frequency of the tuning fork changes as the tip interacts with atoms on the surface without touching the material.
The structure of the aluminum oxide surface was determined by non-contact atomic force microscopy and computer modeling. Credit: TU Wien
Johanna Hütner, who carried out the experiments, explains: “In an ncAFM image, you can see the location of the atoms, but not their chemical identity. We overcame the lack of chemical sensitivity by precisely controlling the tip. By attaching a single oxygen atom to the tip apex, we were able to distinguish between oxygen and aluminum atoms on the surface.”
“The oxygen atom on the tip is repelled by other oxygen atoms on the surface and attracted to the aluminum atoms of the Al2O3 surface. Mapping local repulsion or attraction allowed us to directly visualize the chemical identity of each surface atom.
Restructuring stabilizes the surface without changing its composition
The researchers found that the surface rearranges itself to allow aluminum atoms to penetrate the material and form chemical bonds with oxygen atoms in deeper layers. This rearrangement of the first two atomic layers significantly reduces the energy, effectively stabilizing the structure. Contrary to previous beliefs, the numerical ratio of aluminum and oxygen atoms remains unchanged.
The three-dimensional model of the aluminum oxide surface was optimized using machine learning methods. The main challenge was to match the restructured surface with the underlying crystal.
“The structure is very complex, which gives rise to a large number of possibilities for how the experimentally inaccessible atoms below the surface could be arranged. State-of-the-art machine learning algorithms combined with conventional computational methods allowed us to examine many possibilities and create a stable three-dimensional model of the aluminum oxide surface,” explains Andrea Conti, who carried out the computational modeling.
“Through a collaborative experimental and computational research effort, we have not only solved a long-standing mystery by determining the detailed structure of this enigmatic insulator, but we have also discovered structural design principles applicable to an entire class of materials. Our results pave the way for advances in catalysis, materials science, and other fields,” says Jan Balajka, who led the research.
More information:
Johanna I. Hütner et al., Stoichiometric reconstruction of Al2O3(0001) surface, Science (2024). DOI: 10.1126/science.adq4744. www.science.org/doi/10.1126/science.adq4744
Provided by Vienna University of Technology
Quote:Researchers solve long-standing mystery of alumina surface structure (2024, September 12) retrieved September 12, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.