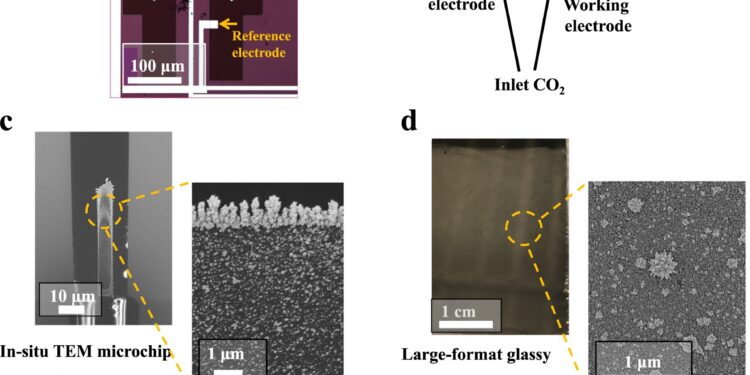
Diagrams of the two COs2 electrolysis cells used in this work. an in situ LP-(S)TEM support Protochips Poseidon consisting of a glassy carbon working electrode decorated with Pd in a microchip electrochemical cell. b Two-compartment electrochemical cell consisting of a large-format Pd-decorated glassy carbon working electrode for electrochemical CO2R activity and selectivity measurements. c SEM images of the working electrode of the in situ TEM microchip covered with electrodeposited Pd particles. d Micrograph of the large-format glassy carbon electrode and SEM image of the electrodeposited Pd particles. e Cyclic voltammetry measurements of electrodeposited Pd particles measured in the in situ TEM microchip electrochemical cell. f Cyclic voltammetry measurements of the electrodeposited Pd particles measured in the two-compartment cell using the large format electrode. Note that all cyclic voltammetry measurements were collected in 0.1 M KHCO saturated with N2.3 at a scan rate of 50 mV/s. Credit: Natural communications (2024). DOI: 10.1038/s41467-024-45096-3
Think of it as nanoscale recycling: a tantalizing electrochemical process that can harvest carbon before it becomes air pollution and restructure it into components of everyday products.
The drive to capture airborne carbon dioxide from industrial waste and turn it into fuel and plastic is gaining momentum after a team of researchers based at McMaster University, in collaboration with experts in computational chemistry from the Danish Technical University in Copenhagen, have discovered precisely how the process works and where it goes wrong.
Their work is published in the journal Natural communications.
Researchers sought to understand why synthetic materials that catalyze and convert carbon dioxide break down too quickly for the process to be practical on an industrial level.
Using extremely powerful magnification equipment at the Canadian Center for Electron Microscopy (CCEM), based on the McMaster campus, the researchers were able to capture the chemical reaction at the nanoscale (billionths of a meter), allowing them to ‘study both the conversion process and understand how the catalyst breaks down under operating conditions.
Lead author Ahmed Abdellah spent years developing the techniques to observe the process, using an electrochemical reactor small enough to operate under the center’s electron microscopes.
“It’s exciting for us that this is the first time that someone can look at both the shapes of these structures and their crystal structures, to see how they evolve on the nanoscale,” says Abdellah, a former doctoral student. student in Drew Higgins’ chemical engineering lab and now a postdoctoral fellow at CCEM.
Higgins, a corresponding author on the paper, hopes the new information will aid the global effort to reduce carbon pollution by removing carbon dioxide from waste streams and recycling it to create useful products that would otherwise be produced from fossil fuels.
“What we found is that catalysts capable of converting carbon dioxide into fuels and chemicals restructure quite quickly under operating conditions. Their structures and properties change, before our eyes,” says Higgins . “This determines how effective they are at converting carbon dioxide and how long they last. The catalysts eventually degrade and stop working and we want to know why they do that and how they do it so we can develop strategies to improve their operational life.”
Abdellah, Higgins and their colleagues hope that they and other researchers around the world can use the research results described in the new paper to extend the life of reactive materials and catalyze the process more efficiently, allowing for the laboratory process to be extended. for commercial use.
Industries such as cement manufacturing, brewing and distillation, as well as chemical refineries, produce large volumes of easily recoverable carbon dioxide, says Higgins, making them likely prime targets for deployment of the technology once that it will have been improved to the point where it will be commercially viable. .
Other less concentrated forms of CO2 in industrial waste would come next.
While it’s a long way off today, Higgins says it’s possible the same technology could become efficient and stable enough to extract carbon dioxide from ambient air as a “feedstock” for fuel and chemicals. useful.
“We’re still a long way off, but progress has been very rapid in this area of research and development over the last five years,” says Higgins. “Ten years ago, people weren’t thinking about this type of conversion, but now we’re starting to see promise. Efficiency and sustainability, however, are simply not high enough yet. Once these bottlenecks are removed, this idea can really take off.”
More information:
Ahmed M. Abdellah et al, Impact of palladium/palladium hydride conversion on electrochemical CO2 reduction via in situ transmission electron microscopy and diffraction, Natural communications (2024). DOI: 10.1038/s41467-024-45096-3
Provided by McMaster University
Quote: Researchers reveal elusive bottleneck holding back global effort to convert carbon dioxide waste into usable products (February 6, 2024) retrieved February 6, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.