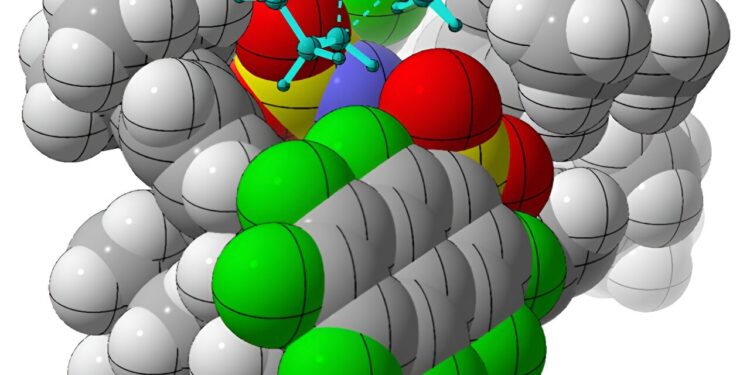
UC Davis chemists use catalysts (shown as gray spheres) to make organic compounds (blue sticks) with specific chirality, or “manity.” Most biological molecules are chiral, including many prescription drugs. This discovery could facilitate the synthesis of pharmaceuticals with the right symmetry. Credit: William DeSnoo/UC Davis
In nature, organic molecules are either left-handed or right-handed, but it is difficult to synthesize molecules with specific “handleability” in the laboratory. Make a drug or enzyme with the wrong “hands” and it simply won’t work. Today, chemists at the University of California, Davis, are getting closer to emulating nature’s chemical efficiencies through computer modeling and physical experimentation.
In a study published on January 10 in Nature, Professor Dean Tantillo, graduate students William DeSnoo and Croix Laconsay and colleagues at the Max Planck Institute in Germany report the successful synthesis of specific chiral (“hand”) molecules using simple hydrocarbon rearrangements in the presence of catalysts complex organics. Most biological compounds, including many prescription drugs, are chiral.
Tantillo and his colleagues hope the results will allow scientists to better harness hydrocarbons for a variety of purposes, such as precursors to drugs and materials.
“The novelty of this paper is that this is really the first time, to my knowledge, that anyone has managed to achieve a carbocation shift that produces one of the mirror image products over the other with high selectivity ” Tantillo said.
Little balls of fat
In chemistry, chirality is a property that refers to a pair of molecules that share an atomic composition but are mirror images of each other. Like your left and right hands, they cannot overlap.
“Synthetic chemists often want to make molecules that appear as a mirror image, but they only want one,” Tantillo said. “For example, if you want to make a drug molecule, you often need one of two chiral forms to selectively bind to a protein or enzyme target.”
Achieving this can be difficult in the lab because, Tantillo says, these molecules often look like “little balls of fat surrounded by a positive charge.”
The fatty nature of these molecules generally makes it difficult to bind a chemical catalyst in one orientation over another due to the lack of charged groups for the catalyst to latch onto.
But researchers have found a solution. Using a chiral organic acid, imidodiphosphorimidate, as a catalyst, the team successfully performed rearrangements of achiral alkenylcycloalkanes, producing chiral molecules of interest called cycloalkenes. Using computational methods, Tantillo and his colleagues deduced how the catalyst selectively produces one chiral form over the other.
Similarities with nature
Tantillo said the resulting reaction is similar to the behavior in nature of enzymes that make hydrocarbon products called terpenes. Part of Tantillo’s research involves mapping terpene reaction pathways using quantum mechanical methods.
“If there are multiple possible paths to a product, then every time you stop at an intermediary on that path, you have the opportunity to get byproducts from that intermediary,” he said. “It is therefore important to know when and why a carbocation wants to stop en route to a given terpene if one is to understand and ultimately rethink terpene-forming enzymes.”
The new method could in principle be exploited to produce both natural and non-natural molecules.
“It’s hard to say if these things will ever be done, but oil is a source of a lot of hydrocarbons, and if you could transform them catalytically into molecules with defined chirality, you would have increased the value of those molecules ” Tantillo said. .
Other co-authors are: Vijay Wakchaure, Markus Leutzsch and Benjamin List, Max Planck Institut für Kohlenforschung, Mülheim an der Ruhr, Germany; and Nobuya Tsuji, Hokkaido University, Sapporo, Japan.
More information:
Vijay N. Wakchaure et al, Catalytic asymmetric cationic shifts of aliphatic hydrocarbons, Nature (2024). DOI: 10.1038/s41586-023-06826-7
Quote: Researchers report successful synthesis of specific chiral molecules using simple hydrocarbon rearrangements (January 10, 2024) retrieved January 10, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.