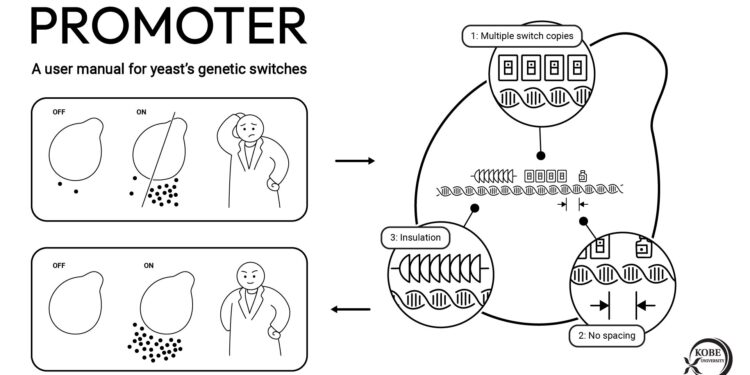
Kobe University bioengineers describe three design principles for efficient and reliably controllable yeast promoters, the genetic switches that regulate artificially introduced genes needed for the cell to produce useful chemicals. First, if researchers not only need large quantities of product, but also need to be able to turn production on or off at will, they should introduce multiple copies of the regulatory elements enabling this within the sponsor. This reduces leaks and increases productivity. Second, the distance between promoter elements should be as small as possible to further improve productivity. And third, the promoter must be isolated from the surrounding DNA by including additional DNA before it to further reduce leakage. Credit: Kobe University
When introducing genes into yeast to make it produce drugs and other useful substances, it is also necessary to reliably turn production on or off. A team from Kobe University has discovered three design principles of gene regulation that provide a flexible guideline for effective control of microbiological production.
DNA is said to be the blueprint of life, telling our cells what to produce. But DNA also contains switches telling these cells when to produce something and how much. Therefore, when introducing new genes into cells to produce useful chemicals such as drugs or raw materials for chemical production, it is also necessary to include a genetic switch, a piece of DNA called “promoter”, which tells cells to start production as soon as possible. necessary.
Tominaga Masahiro, a bioengineer at Kobe University, says: “The problem is that these promoters cannot be used in a plug-and-play way unless researchers have a deep understanding of how they interact with other genetic elements. . promoters to precisely control cell production and achieve their research goal.
Sometimes the production is too low, sometimes it is “leaky”, meaning it cannot be stopped at will. This is particularly true for bioengineered yeasts, whose genetic regulation is more complex than that of bacteria. But this increased complexity also allows its use to produce many useful chemicals.
As experts in modifying yeast cells, Tominaga and his colleagues on the team led by Ishii Jun took a systematic approach to determining how to design effective promoters.
“We had the idea that by carefully describing our process of improving a prototype promoter, we could prepare a ‘user manual’ explaining how to achieve precise and efficient control so that these genetic systems could be more widely used,” Tominaga explains.
In an article now published in the journal Natural communicationsthey describe three design principles for yeast promoters. First, if researchers not only need large quantities of product, but also need to be able to turn production on or off at will, they should introduce multiple copies of the regulatory elements enabling this within the sponsor. This reduces leaks and increases productivity. Second, the distance between promoter elements should be as small as possible to further improve productivity. And third, the promoter must be isolated from the surrounding DNA by including additional DNA before further reducing leakage.
Tominaga says: “We have shown that the performance of a promoter can be improved more than 100-fold by simply modifying its surrounding sequence. This is the first study to clearly propose a solution to the problem of why strong yeast promoters work in some environments and not others. “.
Bioengineers from Kobe University demonstrated the utility of their system by demonstrating the production of two pharmaceutically useful proteins, called “biologics.” Not only could they produce these two biologics in separate yeast strains, but also in the same strain and with the ability to independently control which biologic is produced at any given time.
The latter is important because it has potential applications in hospitals, as the team explains in the study: “In addition to conventional fermentation of single biologics, rapid, single-dose production of multiple biologics with a single yeast strain at the point of caution is crucial for emergencies that require production speed and flexibility rather than purity and productivity.
They also successfully produced a notoriously difficult coronavirus protein that could be used for the production of therapeutics, demonstrating both the utility and flexibility of their design principles.
Tominaga says: “Synthetic biology advocates the creation of new biological functions by rewriting genome sequences. The reality is, however, that we are often confused by the unexpected changes resulting from our edits. We hope our study is the first step toward being able to design each unique base. in the genome with clear intentions. »
More information:
Design strong inducible synthetic promoters in yeast, Natural communications (2024). DOI: 10.1038/s41467-024-54865-z
Provided by Kobe University
Quote: A user manual for yeast genetic switches: Researchers provide three design principles (December 19, 2024) retrieved December 19, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.