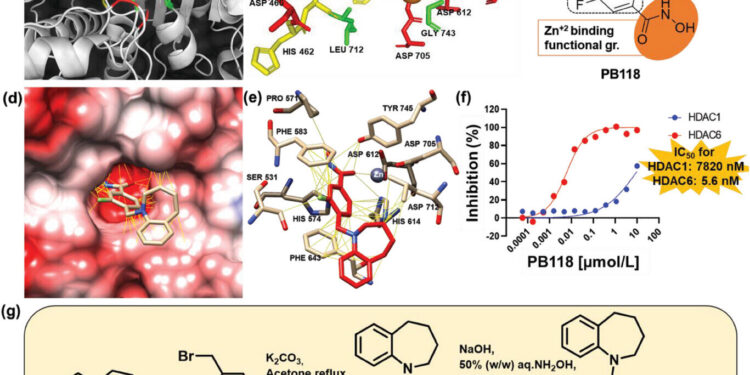
Chemical properties and synthesis of PB118. Credit: Advanced science (2023). DOI: 10.1002/advs.202304545
In people with Alzheimer’s disease (AD), the epigenetic regulator HDAC6 (histone deacetylase 6), which controls many biological processes, is significantly overexpressed in the brain.
Evidence suggests that HDAC6 is closely linked to amyloid and tau pathology, the two main hallmarks of AD.
But it is unclear whether amyloid deposits in AD change with increasing HDAC6 expression as the disease progresses; or whether HDAC6 itself regulates neuroinflammation and other neuropathological changes in AD.
Researchers at Massachusetts General Hospital (MGH), a founding member of the Mass General Brigham Health System, recently used a series of chemical and biological studies in disease-specific cell lines to learn more about the role of HDAC6 in AD and how new treatments designed to inhibit its activity, which could reduce the pathology of the disease. The results of this work are published in Advanced science.
“We still lack effective and safe treatments that can prevent, stop or reverse the progression of AD, largely due to the complex etiology of AD,” said lead author Can (Martin ) Zhang, MD, Ph.D., researcher at the McCance Center for Brain Health at Massachusetts General Hospital. “The collective data suggests we need new angles and alternative approaches.”
Among the cellular functions controlled by HDAC6 are cell proliferation, immune response, stress response, neurological changes, and clearance of misfolded proteins.
When HDAC6 is dysfunctional, abnormal cellular responses result, leading to pathology. HDAC6 has been implicated in several diseases, including cancer and neurodegenerative diseases. Currently, drugs that inhibit HDAC6 are being evaluated in clinical trials, for example for the treatment of cancer.
However, the role of HDAC6 in AD neurodegeneration, as well as that of other epigenetic proteins, is less well explored.
To address this issue, researchers sought to analyze the effects of harmful HDAC6 accumulation in the brain of a mouse model of AD and examined how HDAC6 changed as disease pathology began to progress to a stage early.
“In this work, we saw amyloid deposits and HDAC6 levels become elevated in mouse models of AD as the mice age, suggesting that the two factors may combine to impair amyloid-beta clearance. (Aβ) of the brain and contribute to the adverse effects of the disease,” said lead author Prasenjit Mondal, Ph.D., senior postdoctoral researcher at Massachusetts General Hospital.
The investigators found that their HDAC6 inhibitor, called PB118, cleared beta-amyloid deposits through several mechanisms, including reducing Aβ production, increasing Aβ uptake by the brain, improving of the tubulin/microtubule network disrupted in AD, the regulation of different cytokines and chemokines responsible. for inflammation and significantly reducing AD-associated Tau phosphorylation.
In ongoing research, researchers are developing an inhibitor molecule that binds strongly and exclusively to HDAC6 and not to additional proteins. They are also studying whether the inhibitors have the potential to suppress the neuropathological changes of Alzheimer’s disease in mice.
Additionally, the research team developed a novel positron emission tomography (PET) imaging probe capable of detecting increased HDAC6 levels in the brain and potentially serving as an imaging biomarker for AD.
More information:
Prasenjit Mondal et al, Structure-based discovery of a small molecule inhibitor of histone deacetylase 6 (HDAC6) that significantly reduces Alzheimer’s disease neuropathology, Advanced science (2023). DOI: 10.1002/advs.202304545
Provided by Massachusetts General Hospital
Quote: Researchers identify new small molecule inhibitor for use against Alzheimer’s disease (November 27, 2023) retrieved November 27, 2023 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.